| Cas No.: | 1149348-10-6 |
| Chemical Name: | FGI-106 |
| Synonyms: | FGI-106,FGI106,FGI 106 |
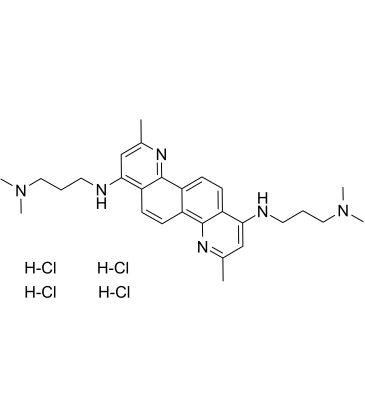
| SMILES: | CC1N=C2C(C=CC3C2=CC=C2C=3N=C(C=C2NCCCN(C)C)C)=C(NCCCN(C)C)C=1.Cl.Cl.Cl.Cl |
| Formula: | C28H42Cl4N6 |
| M.Wt: | 604.49 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Publication: | 1. Kinch MS, et al. Am J Transl Res. 2009 Jan 5;1(1):87-98. 2. De Clercq E. Med Res Rev. 2013 Nov;33(6):1249-77. |
| Description: | FGI-106 tetrahydrochloride is a potent and broad-spectrum inhibitor with inhibitory activity against multiple viruses. FGI-106 tetrahydrochloride is active against Ebola, Rift Valley and Dengue Fever viruses with EC50s of 100 nM, 800 nM and 400-900 nM, respectively. FGI-106 tetrahydrochloride also inhibits non-hemorrhagic fever viruses HCV and HIV-1 with EC50s of 200 nM and 150 nM, respectively[1]. |
| Target: | EC50: 100 nM (Ebola virus), 800 nM (Rift Valley virus), 400-900 nM (Dengue Fever virus), 200 nM (HCV) and 150 nM (HIV-1)[1] |
| In Vivo: | FGI-106 (0.1-5 mg/kg; intraperitoneal injection; treatments on days 2 and 5; C57BL/6 or BALB/c mice) treatment decreases mortality from Zaire EBOV in a dose-dependent manner[1]. Animal Model: Male or female C57BL/6 or BALB/c mice (6-10 weeks of age) injected with EBOV (Ebola virus)[1] Dosage: 0.1 mg/kg, 0.5 mg/kg, 1 mg/kg, 2 mg/kg, 5 mg/kg Administration: Intraperitoneal injection; treatments on days 2 and 5 Result: Decreased mortality from Zaire EBOV in a dose-dependent manner. |
| In Vitro: | Treatment with 2 μM FGI-106 mediated a 4 log reduction in infectious viral titers relative to matched controls, with an EC90 for inhibition of viral killing of host cells (Vero E6 cells) estimated to be 0.6 μM[1]. In cell-based assays, treatment with FGI-106 inhibits viral replication by divergent virus families, including positive and negative-strand RNA viruses[1]. |
| References: | [1]. Aman MJ, et al. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral Res. 2009 Sep;83(3):245-51. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.