| Cas No.: | 1857417-13-0 |
| Chemical Name: | 4-Methyl-1-(1h-Pyrazol-4-Ylmethyl)-5-[(4-{[6-(2,2,2-Trifluoroethyl)thieno[2,3-D]pyrimidin-4-Yl]amino}piperidin-1-Yl)methyl]-1h-Indole-2-Carbonitrile |
| Synonyms: | MI-503;MI 503;MI503;1-((1H-pyrazol-4-yl)methyl)-4-methyl-5-((4-((6-(2,2,2-trifluoroethyl)thieno[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)methyl)-1H-indole-2-carbonitrile;4-Methyl-1-(1h-Pyrazol-4-Ylmethyl)-5-[(4-{[6-(2,2,2-Trifluoroethyl)thieno[2,3-D]pyrimidin-4-Yl]amino}piperidin-1-Yl)methyl]-1h-Indole-2-Carbonitrile;4-methyl-1-(1H-pyrazol-4-ylmethyl)-5-[[4-[[6-(2,2,2-trifluoroethyl)thieno[2,3-d]pyrimidin-4-yl]amino]piperidin-1-yl]methyl]indole-2-carbonitrile;4-Methyl |
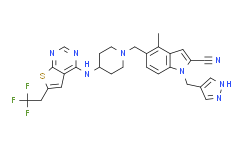
| SMILES: | S1C(C([H])([H])C(F)(F)F)=C([H])C2C1=NC([H])=NC=2N([H])C1([H])C([H])([H])C([H])([H])N(C([H])([H])C2C([H])=C([H])C3=C(C([H])=C(C#N)N3C([H])([H])C3C([H])=NN([H])C=3[H])C=2C([H])([H])[H])C([H])([H])C1([H])[H] |
| Formula: | C28N8F3SH27 |
| M.Wt: | 564.6278 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | MI-503 is a highly potent and orally bioavailable small molecule inhibitor of the menin-mLL interaction. |
| In Vivo: | MI-503 achieves high level in peripheral blood following a single intravenous or oral dose, while also showing high oral bioavailability (75%). MI-503 induces strong inhibition of tumor growth with once daily intraperitoneal (i.p.) administration. Treatment with MI-503 results in an over 80% reduction in MV4;11 tumor volume and complete tumor regression in two mice. Ten consecutive days of treatment with MI-503 results in a marked delay in progression of mLL leukemia in mice and significantly reduces leukemia tumor burden. Treatment with MI-503 and MI-463 leads to markedly reduced expression of Hoxa9 and Meis1, downstream targets of mLL fusion proteins substantially upregulated in mLL leukemias[1]. |
| In Vitro: | MI-503 occupies the F9 and P13 pockets on menin, forming a hydrogen bond with Tyr276, and also extends beyond the P13 pocket to form hydrogen bonds with Trp341 and Glu366. Treatment of murine bone marrow cells (BMC) transformed with the mLL-AF9 oncogene with MI-503 results in substantial growth inhibition, with GI50 of 0.22 μM. The cell growth inhibitory effect of MI-503 is time-dependent, with a pronounced effect achieved after 7–10 days of treatment[1]. |
| Cell Assay: | Leukemia cells are treated with MI-503 or 0.25% DMSO and cultured at 37 °C for 7 days. Media is changed at day 4, viable cell numbers are restored to the original concentration and MI-503 are re-supplied. MTT cell proliferation assay kit is then employed, and plates are read for absorbance at 570 nm using a microplate reader[1]. |
| Animal Administration: | Mice: For efficacy studies in MV4;11 subcutaneous xenograft mice model, 5×106 cells are injected into the 4-6 week old female BALB/c nude mice. Treatment is started when the tumor size reached ~100 mm3. Vehicle (25% DMSO, 25% PEG400, 50% PBS) or compounds (MI-463 or MI-503) are administrated once daily at designated doses using i.p. injections[1]. |
| References: | [1]. Borkin D, et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell. 2015 Apr 13;27(4):589-602. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.