 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
| A1080 | Talacotuzumab-MMAE Featured |

|
|
| A1079 | Lonigutamab-MMAE Featured |

|
|
| DC65455 | Telisotuzumab vedotin Featured |
Telisotuzumab vedotin (Teliso-V, ABBV-399) is an antibody-drug conjugate (ADC) composed of the anti–c-Met humanized monoclonal antibody coupled to the cytotoxic monomethyl auristatin E (MMAE) through a mc-val-cit-PABC type linker. Telisotuzumab vedotin can be used for research on advanced solid tumours.
More description
|

|
| DC65454 | Indusatumab Vedotin Featured |
Indusatumab vedotin (MLN-0264) is an antibody-drug conjugate (ADC) composed of an anti-human GUCY2C (Guanylate cyclase 2C) monoclonal antibody and a anti-mitotic cytotoxic agent, monomethyl auristatin E (MMAE).
More description
|

|
| A1078 | Glembatumumab vedotin Featured |
Glembatumumab vedotin (CDX-011) is an ADC comprising a fully human IgG2 monoclonal antibody (CR011) directed against glycoprotein NMB (GPNMB) and conjugated to the potent tubulinbinding cytotoxic agent MMAE via a protease-sensitive vc linker. Glembatumumab vedotin has potent anticancer effects.
More description
|

|
| A1077 | Ecromeximab-MMAE Featured |

|
|
| DC65464 | Farletuzumab-MMAE Featured |
Farletuzumab-MMAE is an antibody-drug conjugate (ADC) composed of humanized anti-human folate receptor-alpha (FRα) and the cytotoxic agent monomethyl auristatin (MMAE) via a MC-Vc linker. It has a potent antitumor activity.
More description
|

|
| A1076 | Mirvetuximab-MMAE Featured |

|
|
| A1075 | Rosopatamab-MMAE Featured |

|
|
| A1074 | Patritumab-MMAE Featured |

|
|
| A1073 | Trastuzumab vedotin Featured |
Trastuzumab vedotin (MRG002) is an anti-human epidermal growth factor receptor 2 (HER2) antibody-drug conjugate (ADC). Trastuzumab vedotin is composed of a humanized anti-HER2 antibody Trastuzumab (HY-P9907), an enzymatically cleavable peptide-linker Valine-citrulline, a tubulin inhibitor Monomethyl auristatin E (MMAE; HY-15162). Trastuzumab vedotin can be used for the research of HER2-positive breast cancer.
More description
|

|
| A1072 | Cetuximab-MMAE Featured |
Cetuximab-MMAE is an antibody-drug conjugate composed of a anti-EGFR monoclonal antibody and a cytotoxic tubulin polymerase inhibitor conjugated through MC-Vc-PAB (maleimidocaproyl-valine-citrulline-p-aminobenzoyloxycarbonyl) type linker.
More description
|

|
| A1071 | Carotuximab-MMAE Featured |

|
|
| A1070 | Ulocuplumab-MMAE Featured |

|
|
| A1069 | Tusamitamab-MMAE Featured |

|
|
| A1068 | Ladiratuzumab vedotin Featured |
Ladiratuzumab vedotin (SGN-LIV1A) is a LIV-1 targeting antibody drug conjugate (ADC) (IC50: 5.6 nM for LIV-1). Ladiratuzumab vedotin consists of humanized IgG1 monoclonal antibody, MMAE and a protease-cleavable linker. Ladiratuzumab vedotin can drive immunogenic cell death (ICD) to elicit an immune response. Ladiratuzumab vedotin can be used for research of breast cancer.
More description
|

|
| A1067 | Polatuzumab vedotin Featured |
Polatuzumab vedotin solution is an antibody-drug conjugate targeting CD79b. It contains a humanized anti-CD79b IgG1 monoclonal antibody linked to monomethyl auristatin E (MMAE), a potent microtubule inhibitor. The antibody portion is Polatuzumab (HY-P99042), and the drug-linker conjugate for ADC is VcMMAE (HY-15575). Polatuzumab vedotin solution has the potential for the research of Large B-cell lymphomas (LBCL).
More description
|

|
| A1066 | Girentuximab-MMAE Featured |

|
|
| A1065 | Tilvestamab-MMAE Featured |

|
|
| A1064 | Murlentamab-MMAE Featured |

|
|
| A1063 | Praluzatamab-MMAE Featured |

|
|
| A1062 | Anti-BSG2 / CD147 Reference Antibody (HcHAb18-DM1) Featured |

|
|
| A853 | Trabikibart Biosimilar(Anti-CSF2Rb / CD131 Reference Antibody) Featured |
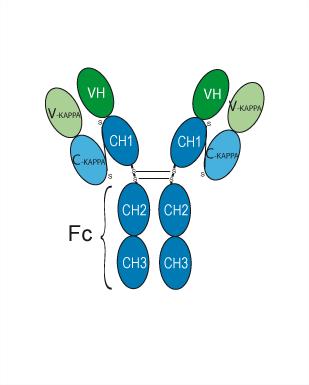
Trabikibart (CSL311), a βc (CSF2RB)-specific, fully human monoclonal antibody, binds to a unique epitope that is specific to the cytokine-binding site of the human βc receptor. Trabikibart has picomolar binding affinity for the human βc receptor. Trabikibart is a potent inhibitor of the combined effects of IL-3, GM-CSF and IL-5 on the survival of eosinophils. Trabikibart has the potential for chronic inflammatory diseases research.
More description
|
|
| A003 | Alirocumab Biosimilar(Anti-PCSK9 Reference Antibody) Featured |
Alirocumab is a human monoclonal antibody that binds to proprotein convertase subtilisin kexin type 9 (PCSK9) and it can reduce cholesterol levels in the blood.
More description
|

|
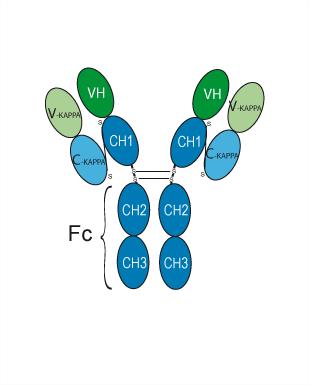
| A014 | Nivolumab Biosimilar(Anti-PDCD1 / PD-1 / CD279 Reference Antibody) Featured |
Nivolumab anti-PD-1) is a genetically engineered fully human immunoglobulin Ig) G4 monoclonal antibody directed against the negative immunoregulatory human cell surface receptor programmed death-1PD-1PCD-1) with immune checkpoint inhibitory and antineoplastic activities.
More description
|
|
| A293 | BMS patent anti-Integrin α5β1 Biosimilar(Anti-Integrin a5b1 (ITGA5 & ITGB1) Reference Antibody) Featured |

|
|
| A226 | Eblasakimab Biosimilar(Anti-IL-13Ra1 / CD213a1 Reference Antibod) Featured |
Eblasakimab (ASLAN004; CSL-334) is a human IgG4 antibody that specifically targets IL13RA1 and is primarily expressed by CHO-K1 cells.
More description
|
.png)
|
| A1061 | Osemitamab Biosimilar(Anti-CLDN18.2 Reference Antibody) Featured |
Osemitamab is an IgG1 antibody targeting to human claudin-18.2. Osemitamab consists of human-Mus musculus monoclonal TST001 γ1-chain, disulfide with human-Mus musculus monoclonal TST001 κ-chain, dimer (ACI). Osemitamab in combination with Capecitabine (HY-B0016) and Oxaliplatin (HY-17371), can be used for G/GEJ cancer study.
More description
|

|
| A1060 | Muromonab Biosimilar(Anti-CD3 Reference Antibody) Featured |
Muromonab is a monoclonal antibody targeting the CD3 receptor. Muromonab can block all cytotoxic T cell function. Muromonab also as an immunosuppressant agent given to reduce acute solid organ transplant rejection.
More description
|

|
| A1059 | Atoltivimab Biosimilar(Anti-EBOV Reference Antibody) Featured |
Atoltivimab (REGN3470), or maftivimab/odesivimab (Inmazeb) is the first Food and agent Administration (FDA)-approved monoclonal antibody to target Zaire ebolavirus (EBOVs) infection.
More description
|

|