| Cas No.: | 2414674-71-6 |
| Chemical Name: | SUVN-911 HCl |
| Synonyms: | 2414674-70-5(free base),SUVN-911,SUVN911,SUVN 911 |
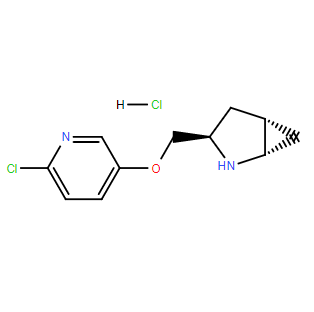
| SMILES: | [C@H]12N[C@H](COC3C=NC(Cl)=CC=3)C[C@H]1C2.[H]Cl |
| Formula: | C11H14Cl2N2O |
| M.Wt: | 261.15 |
| Purity: | >97% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | SUVN-911 is a potent, selective, brain penetrated and orally bioavailable neuronal nicotinic acetylcholine α4β2 receptor antagonist, with a Ki of 1.5 nM. SUVN-911 has antidepressant activity[1]. |
| Target: | Ki: 1.5 nM (α4β2 receptor)[1] |
| In Vivo: | SUVN-911 is devoid of cardiovascular and gastrointestinal side effects[1]. SUVN-911 (1.0-10.0 mg/kg; p.o.; daily; for 3 days) shows significant antidepressant effects[1]. SUVN-911 shows metabolic stability in rats[1]. SUVN-911 (3 mg/kg; p.o.) has shown high oral exposures, longer half-lives and adequate brain penetration in Wistar rats[1]. Animal Model: Male Wistar rats (180-230 g)[1] Dosage: 1 mg/kg, 3 mg/kg, 10.0 mg/kg Administration: Oral administration, daily, for 3 days Result: Showed antidepressant like activity with no signs of tachyphylaxis. Animal Model: Male Wistar rats (225 ± 25 gm)[1] Dosage: 3 mg/kg (Pharmacokinetic Analysis) Administration: Oral administration Result: AUC=3507 ng*h/mL, T1/2=3.34 hours. |
| In Vitro: | SUVN-911 displays high selectivity for α4β2 over α3β4 nAChR[1]. SUVN-911 shows good selectivity against over 70 receptors which includes GPCRs, ion channels, enzymes, peptides, steroids, second messengers, growth factors and prostaglandins[1]. |
| References: | [1]. Ramakrishna Nirogi, et al. Discovery and Development of 3-(6-Chloropyridine-3-yloxymethyl)-2-azabicyclo[3.1.0]hexane Hydrochloride (SUVN-911): A Novel, Potent, Selective, and Orally Active Neuronal Nicotinic Acetylcholine α4β2 Receptor Antagonist for the Treatment of Depression. J Med Chem. 2020 Mar 26;63(6):2833-2853. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.