 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
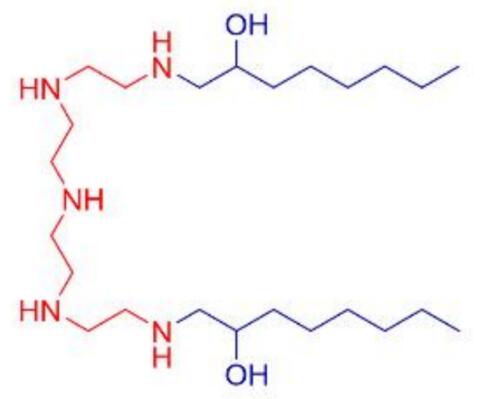
| DC60578 | TE-EP8-S |
TE-EP8-S is a single-component, ionizable cationic lipid designed specifically for the targeted delivery of mRNA to T cells within the spleen. This innovative lipid formulation enhances the efficiency and precision of mRNA-based therapies by ensuring optimal cellular uptake and expression in immune cells. Its unique structure and properties make it a promising tool for advancing immunotherapeutic applications.
More description
|
|
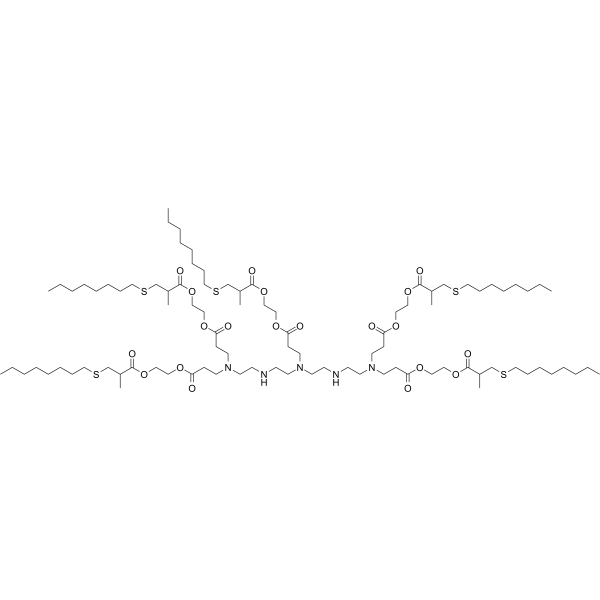
| DC49907 | 5A2-SC8 Featured |
5A2-SC8 is a dendrimer for miRNA delivery to late-stage liver tumors with low hepatotoxicity. 5A2-SC8 shows potent EC50 < 0.02 mg/kg (siRNA against FVII (siFVII)) in dose-response experiments, and well tolerated in separate toxicity studies in chronically ill mice bearing MYC-driven tumors. 5A2-SC8 is a degradable lipid-like compound (ester-based dendrimer) for small RNAs delivery.5A2-SC8, was obtained by screening a large library of more than 1500 ester-based dendrimers
containing ionizable amino groups, which have three
tertiary amine heads and five lipid tails. Based on this library,
the in vitro transfection efficiency of different formulations of
5A2-SC8 iLNPs was evaluated, discovering the optimal formulation
(5A2-SC8, DOPE, cholesterol, PEG at a molar ratio of
15:15:30:3) of 5A2-SC8 iLNPs for delivering fumarylacetoacetate
hydrolase (FAH) mRNA to liver.After the intravenous injection
via tail, the model mice of hepatorenal tyrosinemia type I
had strong FAH protein expression, which prevented
body weight loss and increased the survival rate of hepatorenal
tyrosinemia mice . In addition to introducing utility of
5A2-SC8 iLNPs for the therapeutic intervention, the 5A2-SC8
iLNPs containing DOTAP have been used to establish complex
mouse models via intravenous injection, including in situ liverspecific
cancer model and in situ lung-specific cancer model.
Based on this iLNPs delivery system, 5A2-SC8 induced model
construction method overcomes the time-consuming and costly
disadvantages of traditional animal models establishing methods,
including transgenesis and gene engineering in embryonic
stem cells.
More description
|
|
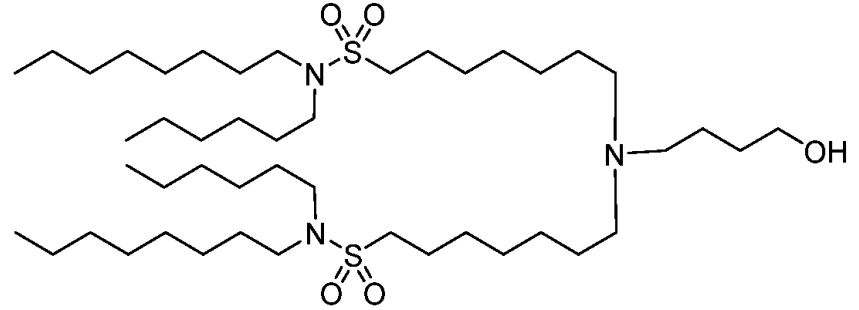
| DC67281 | BNT-51 Featured |
BNT-51 is an ionizable thiolipid developed by Biontech, characterized by its sulfur-containing moieties and a multiarm dendron-like architecture. Synthesized via reactions between amine-containing compounds and sulfur-based halides or sulfonates, it forms stable lipid nanoparticles (LNPs) optimized for mRNA delivery. The LNPs exhibit uniform particle size (80–100 nm, PDI <0.2), near-neutral zeta potential, and high mRNA encapsulation efficiency (>90%), while maintaining payload integrity through freeze-thaw cycles and extended storage. In vitro, BNT-51 demonstrates low cytotoxicity (>80% cell viability in C2C12, HepG2, and HEK293 cells) and superior transfection efficiency compared to conventional lipids, particularly in immune cells such as CD4+/CD8+ T cells within PBMCs. Its modular design allows integration of stealth lipids (e.g., PEG or vitamin E derivatives) to prolong circulation time and minimize immune activation, as evidenced by low hemolysis and complement activation risks. In vivo, BNT-51-based LNPs enable targeted mRNA delivery to splenic macrophages, achieving potent genome editing (e.g., Cre mRNA) and therapeutic protein expression (e.g., BACH1) in preclinical models. With its tunable structure, robust stability, and cell-specific tropism, BNT-51 holds promise for advancing mRNA therapeutics in gene editing, cancer immunotherapy, and regenerative medicine, offering a versatile platform for next-generation nanomedicine.
More description
|
|
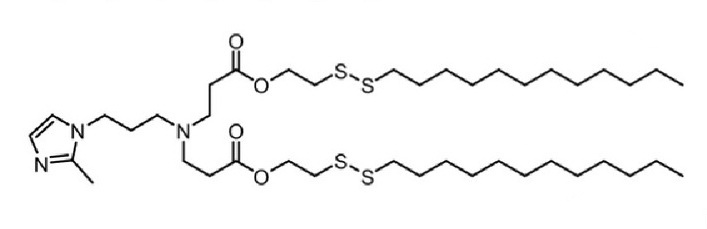
| DC60495 | 9322-O16B Featured |
9322-O16B is a lipidoid for the efficient delivery of antiCD19 mRNA CAR to murine primary macrophages. LNP 9322-O16B is more efficient than delivery with lipofectamine 2000 (LPF2K) or MC3.
More description
|
|
| DC67557 | Tidal Lipid 40 |
Tidal Lipid 40is an ionizable cationic lipid engineered to deliver RNA with high precision to immune cells like macrophages. Based on Tidal Therapeutics' patent US 20250205169A1, Its pH-responsive design shifts from a +8 mV charge at pH 5.5 (enabling endosomal escape) to near-neutral at pH 7.4 (reducing off -target binding), ensuring efficient intracellular release while maintaining blood stability. In lipid nanoparticles, Lipid 40 achieves 65% transfection efficiency in human macrophages—surpassing benchmarks like ALC-0315—and protects >95% of RNA payloads from degradation. Critically, it maintains particle integrity after freeze-thaw cycles with minimal size drift (<5 nm) and excels in in vivo targeting, driving potent gene expression in tumor-associated macrophages while avoiding liver/spleen accumulation. This combination of precision delivery, stability, and low toxicity makes it ideal for immunotherapies, such as reprogramming M2 macrophages to anti-tumor M1 states.
More description
|

|
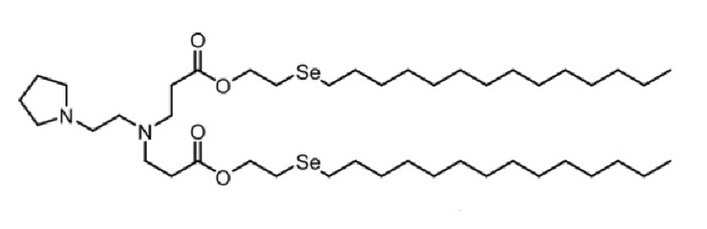
| DC60494 | 76-O17Se |
76-O17Se is a lipidoid for the efficient delivery of antiCD19 mRNA CAR to murine primary macrophages. 76-O17Se is more efficient than delivery with lipofectamine 2000 (LPF2K) or MC3
More description
|
|
| DC67544 | HCQ Lipid 4(HCQ-4) |
HCQ-4 is a rationally engineered ionizable lipid derived from hydroxychloroquine (HCQ), featuring a ditetradecylamine-derived twin-C14 saturated hydrocarbon tail linked to the HCQ headgroup via a succinic acid spacer. Synthesized through a three-step route involving HCQ deprotonation, ditetradecylamine carboxylation, and EDC/DMAP-mediated amidation, this lipid forms the core of optimized lipid nanoparticles (LNPs) at a molar ratio of 60:10:40:0.5 (HCQ-4:DOPE:cholesterol:DMG PEG2000). The structure enables dual functionality: (1) Spleen-selective mRNA delivery (2.3-fold higher splenic vs. hepatic transfection) via 80-100 nm particle size, near-neutral charge (-3 mV), and low PEG density, facilitating immune cell uptake; (2) Tumor microenvironment modulation through HCQ-mediated repolarization of M2 macrophages to antitumor M1 phenotype (iNOS+ cells ↑2.5-fold, CD206+ cells ↓60%). This bifunctional design synergistically enhances mRNA cancer vaccine efficacy, demonstrating superior prophylactic/therapeutic antitumor activity and antimetastatic effects compared to clinical benchmarks like MC-3 LNP.
More description
|

|
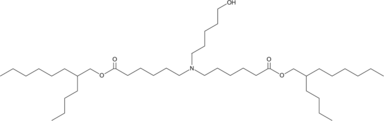
| DC67450 | A28-C6B2 |
A28-C6B2 is an ionizable lipid (pKa 6.43) designed for mRNA encapsulation in lipid nanoparticles (LNPs). Following intravenous injection in mice, these LNPs exhibit spleen-selective accumulation, particularly localizing in F4/80+ macrophages and CD11c+ dendritic cells, with moderate uptake by T lymphocytes.
More description
|
|
| DC80065 | 113-O12B Featured |
113-O12B is a disulfide bond-containing ionizable cationic lipidoid. 113-O12B LNP, an LN-targeting LNP delivery system, is developed for a mRNA cancer vaccine. The 113-O12B/mRNA shows enhanced expression in APCs compared with ALC-0315/mRNA, indicating the LN-specific targeting ability.
More description
|
.gif)
|
| DC53130 | 93-O17S Featured |
93-O17S is an imidazole-based synthetic lipidoid for in vivo mRNA delivery. Lipid nanoparticles (LNPs) with 93-O17S promotes both the cross-presentation of tumor antigens and the intracellular delivery of cGAMP (STING agonist).
More description
|

|
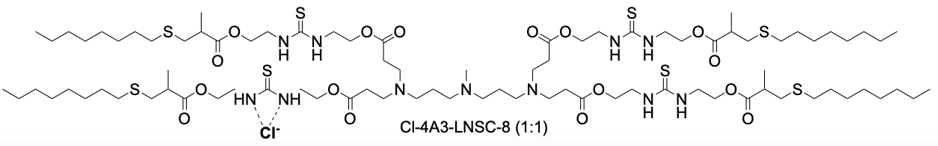
| DC67989 | Cl-4A3-LNSC8 Featured |
Cl-4A3-LNSC8 represents a novel class of thiourea-functionalized ionizable lipids engineered for selective organ-targeted mRNA delivery. Its core innovation lies in an anion-coordination strategy, where the parent lipid, 4A3-LNSC8, binds chloride ions (Cl⁻) via hydrogen-bonding interactions with its thiourea groups. This binding event is not merely structural but functionally critical, as it induces a significant shift in the surface pKa of the resulting lipid nanoparticles (LNPs) from approximately 5.54 to 8.79. This pKa modulation is the key mechanism that redirects the organotropism of the LNPs upon systemic administration. While the unmodified 4A3-LNSC8 LNPs preferentially deliver mRNA to the liver, Cl-4A3-LNSC8 LNPs effectivelyreprogram this tropism, enabling highly efficient mRNA delivery to secondary lymphoid organs (SLOs), particularly the spleen and lymph nodes. This platform demonstrates remarkable efficacy, achieving up to 65.7% gene editing efficiency in splenic macrophages in vivo, significantly outperforming benchmark delivery systems. Furthermore, by leveraging the coordination with different halides, such as iodine for computed tomography (CT) contrast, the system can be adapted for dual-modal theranostic applications, enabling simultaneous lymphatic metastasis imaging and therapeutic mRNA delivery.
More description
|
|
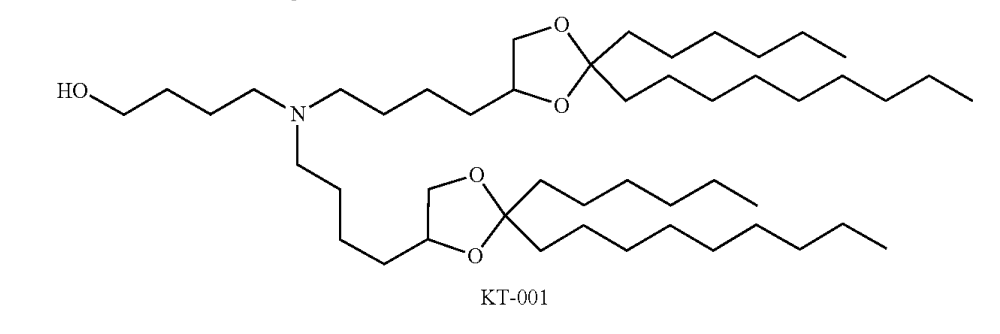
| DC67988 | KT-001 Featured |
KT-001 is a novel ionizable cationic lipid disclosed in patent US 2026/0007612 A1
More description
|
|
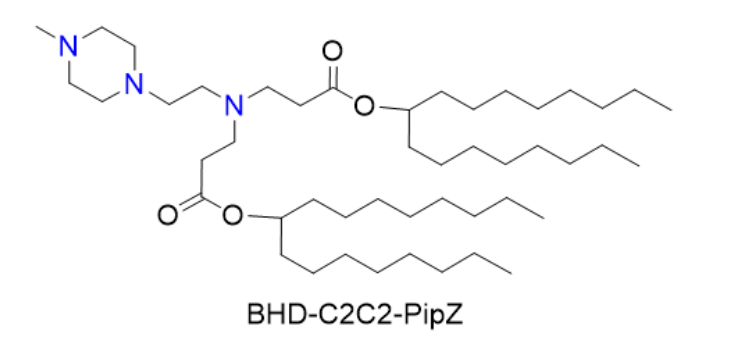
| DC60917 | BHD-C2C2-PipZ Featured |
BHD-C2C2-PipZ, as an efficient ionizable cationic lipid, achieves high encapsulation efficiency and controllable release of mRNA through its unique chemical structure. In PEG-free 3P-LNPs, its electrostatic interaction with tripolyphosphate successfully replaces the steric stabilization effect of traditional PEG, offering a new strategy to circumvent PEG immunogenicity. Its hepatic distribution pattern further indicates that LNP design should take into account the heterogeneity of the organ microenvironment.
More description
|
|
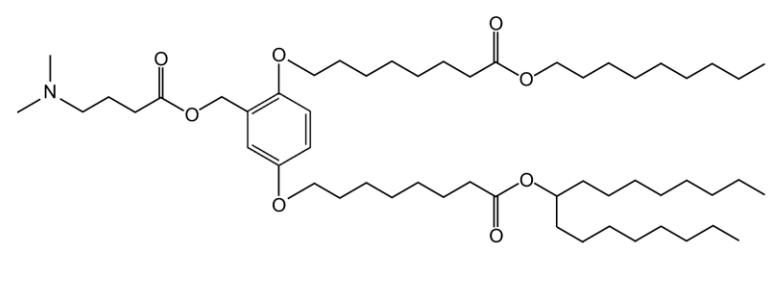
| DC67983 | XH-07 Featured |
XH-07 is an innovative ionizable cationic lipid that forms the backbone of the JCXH-211 lipid nanoparticle (LNP) delivery system. This complex is engineered to encapsulate and deliver self-replicating RNA (srRNA) encoding interleukin-12 (IL-12), a potent immunostimulatory cytokine. The LNP formulation featuring XH-07 exhibits optimal physicochemical properties, such as a mean particle size of approximately 82.12 nm with low polydispersity, and a near-neutral zeta potential around -3.181 mV, which facilitates stable circulation and efficient cellular uptake upon intravenous administration. Upon delivery, the srRNA leverages the host cell's machinery to produce sustained levels of IL-12p70, as demonstrated in B16F10 tumor-bearing mice, where a single dose led to peak cytokine production in sera and tumors. This induced IL-12 expression activates T cells and NK cells, generating a robust antitumor response. In murine models of melanoma and breast cancer, JCXH-211 monotherapy resulted in significant tumor regression and complete responses in some subjects, and it synergized with anti-PD-1 therapy to enhance efficacy. Importantly, the safety profile was acceptable, with transient liver enzyme elevations in mice that normalized quickly, and no significant adverse events in cynomolgus monkeys after repeated dosing, as evidenced by stable clinical observations and pathology tests. Thus, XH-07 is pivotal for enabling the safe and effective delivery of IL-12 encoding RNA, positioning JCXH-211 as a promising cancer immunotherapy.
More description
|
|
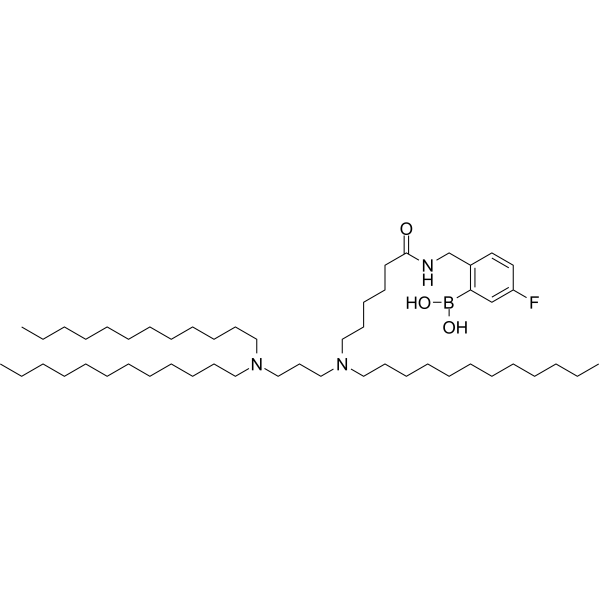
| DC67981 | Diamino lipid DAL4 Featured |
Diamino lipid DAL4 is diamino lipid for the preparation of lipid nanoparticles (LNPs) encapsulated with mRNAs encoding cytokines including IL-12, IL-27 and GM-CSF. Diamino lipid DAL4 delivers mRNA to tumor cells to exert anti-tumor activity.
More description
|
|
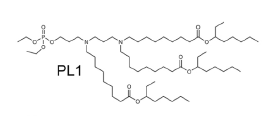
| DC80068 | LIPID PL1 |
PL1 is a novel biomimetic phospholipid. PL1 nanoparticle delivery of costimulatory
receptor CD137 mRNA improved the immunotherapy with an
anti-CD137 Ab to some extent in both tumor models with better
results obtained in the B16F10 melanoma model as compared to
the A20 lymphoma model.
More description
|
|
| DC67563 | S-Ac7-DOg Featured |
S-Ac7-DOg is an ionizable lipid engineered for optimized mRNA delivery to the retina, featuring a sulfur-based ester bond (S-Ac) and dual oleyl glyceride chains (DOg). Its pKa (~6.74) is finely tuned to enhance endosomal escape in acidic environments, enabling efficient cytosolic mRNA release. Unlike traditional lipids (e.g., C12-200, MC3), S-Ac7-DOg incorporates biodegradable ester linkages that hydrolyze intracellularly, minimizing lipid accumulation and reducing innate immune activation.
In vitro, S-Ac7-DOg LNPs achieved >80% transfection efficiency in retinal cells (ARPE-19, MIO-M1) with negligible cytokine secretion, outperforming MC3 and rivaling C12-200 while avoiding the latter’s high immunogenicity. In vivo, intravitreal delivery in mice showed robust protein expression in the optic nerve head (ONH) and Müller glia (75–100% of eyes), sustained for ≥7 days. Critically, it induced the lowest immunogenicity among tested lipids: minimal leukocyte infiltration (<1.5-fold vs. PBS), no microglial reactivity, and reduced GFAP upregulation.
More description
|

|
| DC33580 | DODMA Featured |
DODMA, also known as MBN 305A is a a cationic lipid containing the unsaturated long-chain (18:1) oleic acid inserted at both the sn-1 and sn-2 positions. It has been used in the composition of lipospomes formulated as stable nucleic acid lipid particles that can encapsulate siRNA or other small molecules to be used for drug delivery
More description
|

|
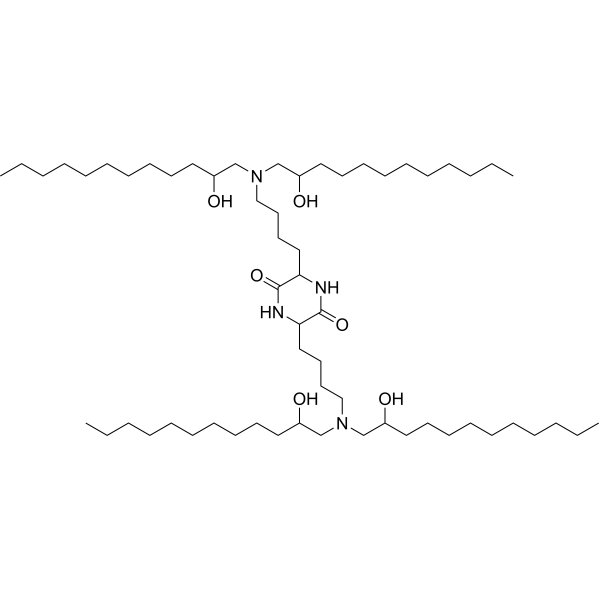
| DC49882 | CKK-E12 Featured |
CKK-E12 is a ionizable lipid in combination with other lipids make up the lipid nanoparticles which are used to deliver RNA-based therapeutics. cKK-E12 was highly selective toward liver parenchymal cell in vivo.Multitail lipids usually have three or more tails and tend to form
more cone-shaped structures due to the increase of tail crosssection,
which enhances the endosome escape and mRNA
delivery efficiency.CKK-E12 is an ionizable lipid with four
lipid tails and diketopiperazine core-based head. It has shown
excellent efficiency in delivering CRISPR-Cas9 mRNA and
sgRNA.cKK-E12 iLNPs encapsulated mRNA was used to
investigate the effect of Toll-like receptor 4 (TLR4) on iLNPsmediated
mRNA delivery, and it has been demonstrated that
the targeting, safety and efficacy of iLNPs are closely related
to disease state. In other words, even though iLNP delivers
therapeutic mRNA to a given cell type in one disease state, it
is not guaranteed to deliver mRNA to the same cell type in
another disease. As same as MC3 and C12-200, CKK-E12 is also
used to be a positive control ionizable lipid when exploiting new
ionizable lipids.
More description
|
|
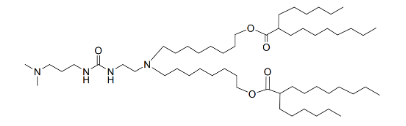
| DC67280 | Lipid 35 |
Lipid 35 is an novel ionizable lipid designed to enhance the delivery of mRNA to specific tissues, particularly the lungs.Lipid 35 demonstrates superior chemical stability, especially in storage conditions. This stability ensures that the lipid maintains its integrity over extended periods, making it ideal for long-term storage and large-scale production.Lipid 35 exhibits high transfection efficiency in various cell types, including nonimmune cells, endothelial cells, and epithelial cells. Lipid 35 has demonstrated excellent biocompatibility and safety in preclinical studies. It does not cause significant liver damage or adverse immune responses, making it a safer alternative for therapeutic applications.
More description
|
|
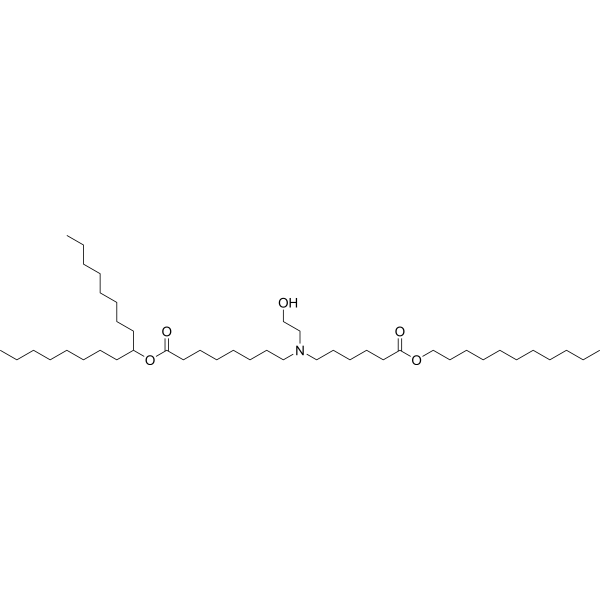
| DC52025 | SM-102 Featured |
SM-102 is an ionizable amino lipid that has been used in combination with other lipids in the formation of lipid nanoparticles.Administration of luciferase mRNA in SM-102-containing lipid nanoparticles induces hepatic luciferase expression in mice. Formulations containing SM-102 have been used in the development of lipid nanoparticles for delivery of mRNA-based vaccines.
More description
|
|
| DC10800 | DLin-MC3-DMA Featured |
D-Lin-MC3-DMA(MC3) is the most potent cationic lipid that has been synthesized for Lipid nanoparticles (LNPs) to deliver the siRNA.
More description
|

|
| DC86120 | EA-PIP(LIPID 10) Featured |
Lipid 10 is a novel ionizable cationic lipid be used for delivery of therapeutic RNA to the Bone Marrow in Multiple Myeloma Using CD38-Targeted with Lipid 10-LNP.
More description
|

|
| DC71417 | YSK 05 Featured |
YSK 05 is a pH-sensitive cationic lipid. YSK 05 improves the intracellular trafficking of non-viral vectors. YSK 05-MEND shows significantly good gene silencing activity and hemolytic activity. YSK 05 overcomes the suppression of endosomal escape by PEGylation. YSK 05 effectively enhances siRNA delivery both in vitro and in vivo.
More description
|

|
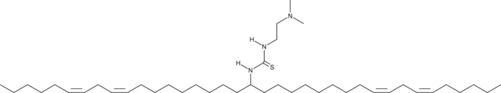
| DC67458 | DMT7 |
DMT7 (pKa 6.5) is an ionizable cationic lipid engineered for co-delivery of mRNA and immunomodulators via LNPs. In 4T1 breast cancer metastasis models, DMT7 LNPs carrying IL-12 mRNA and STING agonist MSA-2 significantly reduce tumor burden and pulmonary metastases while modulating T cell populations. The formulation demonstrates broad immunotherapeutic effects in melanoma models, shifting tumor macrophages toward the M1 phenotype, reducing Tregs, and elevating pro-inflammatory cytokines (IL-12, IL-2, TNF-α, IFN-γ).
More description
|
|
| DC12381 | DLin-KC2-DMA Featured |
DLin-KC2-DMA is a highly potent ionizable lipid used in the formulation of lipid nanoparticles (LNPs) for the delivery of siRNA. It represents a significant advancement over earlier generations of lipids, such as DLin-DMA, due to its dramatically improved gene silencing efficiency.
More description
|
|
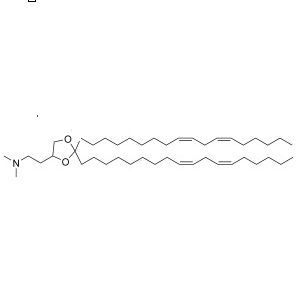
| DC67984 | L31(Lipid 31) Featured |
L31 is identified as a novel, proprietary ionizable cationic lipid that serves as the critical functional component within lipid nanoparticles (LNPs) engineered for CRISPR/Cas9 genome editing in head and neck squamous cell carcinoma (HNSCC). It was selected from a screened library of lipids for its superior performance. LNPs formulated with L31 exhibited excellent physicochemical properties, including a uniform size of 80-100 nm, low polydispersity, and high encapsulation efficiency (>85%) for both Cas9 mRNA and sgRNA. In vitro, L31-based LNPs demonstrated outstanding therapeutic efficacy, achieving approximately 68% gene editing of the oncogene SOX2 and an 88% reduction in cancer cell viability.For in vivo applications, L31-LNPs were further functionalized with anti-EGFR antibodies using the ASSET linker strategy to create targeted nanoparticles (tLNPs). This modification enhanced specific uptake by tumor cells. In a xenograft mouse model, intratumoral injection of these targeted L31-cLNPs co-encapsulating Cas9 mRNA and sgSOX2 led to potent tumor growth inhibition (90%) and a significant increase in survival, with tumor disappearance observed in half of the treated mice. In conclusion, L31 is a highly efficient ionizable lipid that forms the foundation of a potent targeted LNP platform for precise CRISPR-based cancer therapy against solid tumors.
More description
|
.png)
|
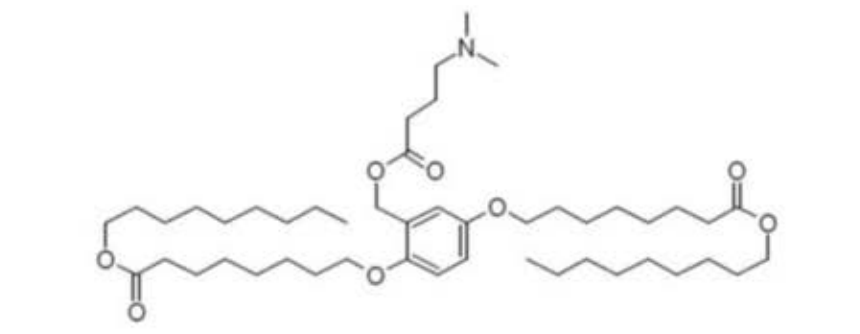
| DC67538 | XH-04 Featured |
XH-04 is an ionizable lipid engineered for advanced mRNA delivery developed by JiaChen West Lake Biotech. Its core structure features a central benzene ring with asymmetric hydrophobic tails (C9-C10 chains) and pH-responsive tertiary amines that enable efficient mRNA encapsulation and endosomal escape. As detailed in CN113993839A, XH04 outperforms industry benchmarks (e.g., MC3 lipid), boosting protein expression by >10-fold in BHK cells. In PCT/CN2024/121624, JiaChen further demonstrated its utility in lung-targeted LNPs (tLNP/tLCNP). When combined with cationic lipids (e.g., DOTMA at 2:1 molar ratio), XH 04 redirects >80% of mRNA delivery to murine lungs—overcoming liver tropism—while maintaining low toxicity. The lipid’s benzenic core and optimized alkyl chain geometry (patent claims 1-9) are credited for enhanced endosomal disruption and mRNA release kinetics. JiaChen’s innovations position XH-04 as a cornerstone for next-generation mRNA therapeutics.
More description
|
|
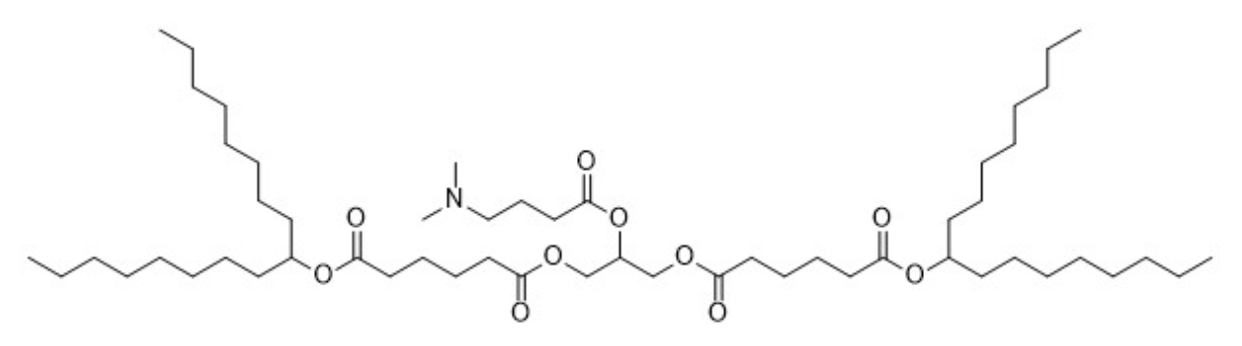
| DC67449 | Lipid TG4C Featured |
TG4C is an ionizable cationic lipid (pKa 6.71) optimized for mRNA delivery via lipid nanoparticles (LNPs). When formulated into LNPs carrying human EPO mRNA, it significantly elevates serum EPO levels in mice. Furthermore, aerosolized TG4C-based LNPs containing HGF mRNA demonstrate therapeutic potential in pulmonary emphysema models, showing reduced inflammatory cytokines (IL-1β, IL-6, TNF-α) in bronchoalveolar lavage fluid after elastase-induced lung injury.
More description
|
|
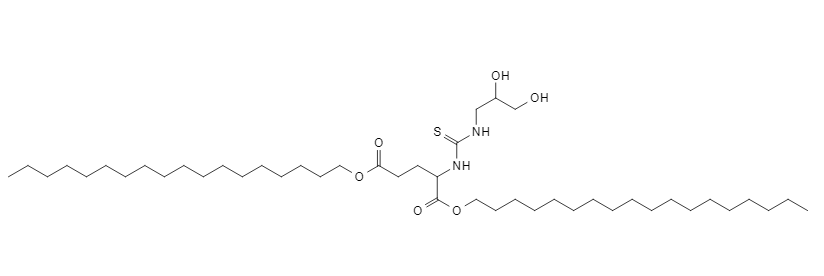
| DC60537 | C18 NC-TNP Featured |
NC-TNP (noncationic thiourea lipids nanoparticles) could compress mRNA by strong hydrogen bonds interaction between thiourea groups of NC-TNP and the phosphate groups of mRNA. NC-TNP could escape the recycling pathway to inhibit the egress of internalized nanoparticles from the intracellular compartment to the extracellular milieu. NC-TNP-encapsulated mRNA shows higher gene transfection efficiency in vitro and in vivo than mRNA-LNP formulation. NC-TNP also shows spleen targeting delivery ability with higher accumulation ratio (spleen/liver), compared with traditional LNP.The C18 non-cationic thiourea lipid self-assembles into ~100 nm nanoparticles with neutral surface charge, utilizing strong hydrogen bonding between its thiourea groups and mRNA phosphate groups for efficient mRNA complexation. This delivery system demonstrates significantly enhanced EGFP expression efficiency—2.3-fold higher than standard C6/C12 formulations—in DC2.4, B16, and 4T1 cells, while sustaining luciferase activity for over 20 days post-subcutaneous injection. It exhibits exceptional stability, maintaining >94% mRNA integrity and <10% particle size variation after 30-day lyophilized storage. Importantly, the nanoparticles show pronounced spleen-targeting capability with 20-fold greater accumulation in the spleen versus liver, effectively activating twice the level of antigen-specific CD8⁺ T cells. Critically, the system avoids cationic lipid-associated toxicity, inducing no detectable IL-6/CXCL10 inflammation and causing no histopathological damage in cardiac or splenic tissues, thus establishing a novel high-efficacy, low-toxicity mRNA delivery platform.
More description
|
|