 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
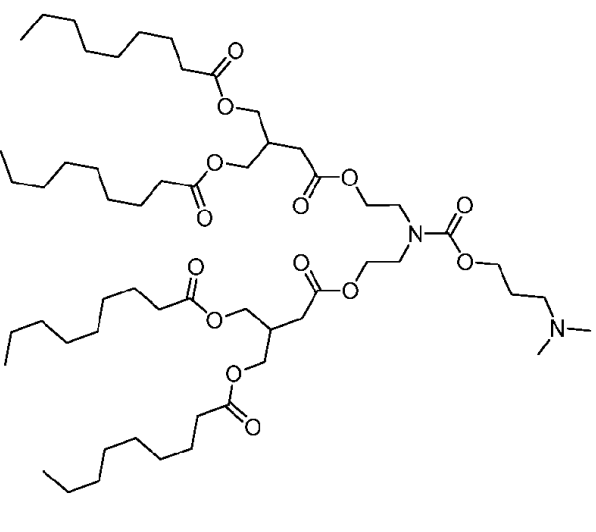
| DC60878 | Lipid A-12 Featured |
Lipid A-12 is an ionizable cationic lipid from Capstan Therapeutics and a close analog of CICL-1 (L829). The key structural distinction is in the headgroup spacer length, where the value of 'n' is 1 in A-12, compared to 0 in CICL-1 (L829).
More description
|
|
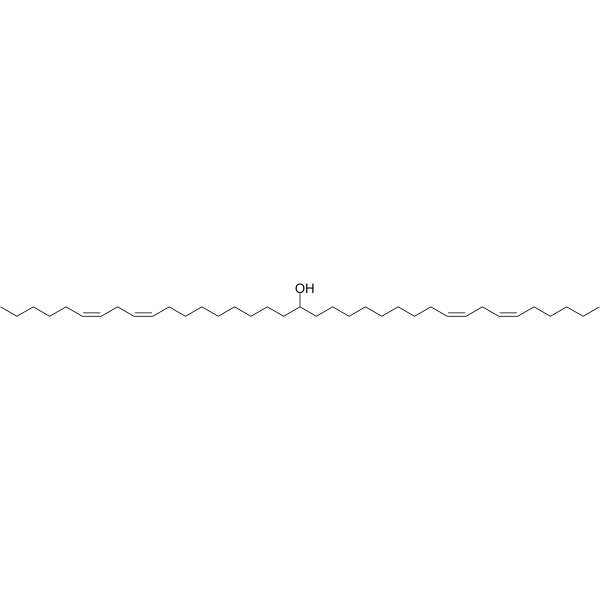
| DC71687 | Dlin-MeOH Featured |
Dlin-MeOH is a lipid product for use in drug delivery systems.
More description
|
|
| DC67602 | ILB-3132(E12LA6B603) Featured |
E12LA6B603(ILB3132,ILB-3132) is a novel ionizable amino lipid disclosed in patent WO2024198497A1, developed by MagicRNA, representing a highly efficient component for lipid nanoparticle (LNP) delivery systems.When formulated into LNPs, E12LA6B603 LNP achieves a remarkable 98.26% encapsulation efficiency for mRNA. It mediates superior in vitro transfection in dendritic cells (1.8E+05 intensity) and demonstrates best-in-class in vivo protein expression after intramuscular injection (2.2E+09 intensity). Most notably, in a B16-OVA melanoma model, therapeutic OVA-mRNA vaccines delivered by E12LA6B603 LNPs induced 100% complete tumor regression, highlighting its superior efficacy over benchmarks like DLin-MC3 and SM-102. Its biodegradable ester linkages and balanced structure make it a promising, potent candidate for next-generation mRNA vaccines and therapeutics.
More description
|

|
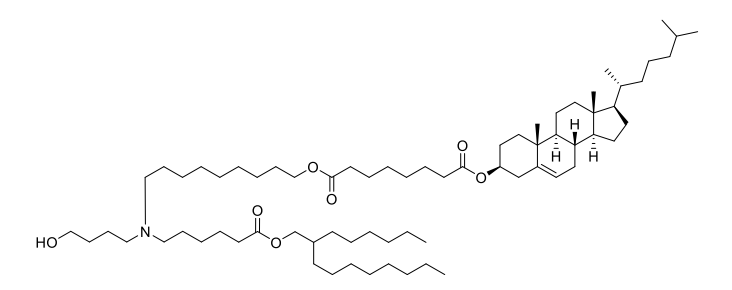
| DC67617 | iChol15-C4A2 |
iChol15-C4A2 is a groundbreaking ionizable cholesteryl lipid, expertly designed to overcome the primary challenge of liver-centric accumulation in mRNA therapeutics. Its innovative "two-in-one" structure seamlessly integrates cholesterol with an ionizable headgroup, enabling the formation of stable, three-component Lipid Nanoparticles (Tc-LNPs).The key advantage of Tc-LNPs formulated with iChol15-CA2 is their significantly reduced adsorption of Apolipoprotein E (ApoE).This unique property directly attenuates ApoE/LDLR-mediated uptake by liver cells, dramatically shifting biodistribution toward extrahepatic tissues. Peer-validated research demonstrates a remarkable 20-50 fold increase in the spleen-to-liver mRNA expression ratio compared to standard LNPs like ALC-0315, unlocking unparalleled potential for targeting the immune system.
Beyond its superior targeting capability, iChol15-C4A2 ensures high mRNA encapsulation efficiency, excellent colloidal stability, and proven biocompatibility. It offers a powerful, off-the-shelf solution to advance next-generation mRNA applications, from innovative vaccines and cancer immunotherapies to treatments for splenic disorders. Discover how iChol15-C4A2 can transform your delivery platform.
More description
|
|
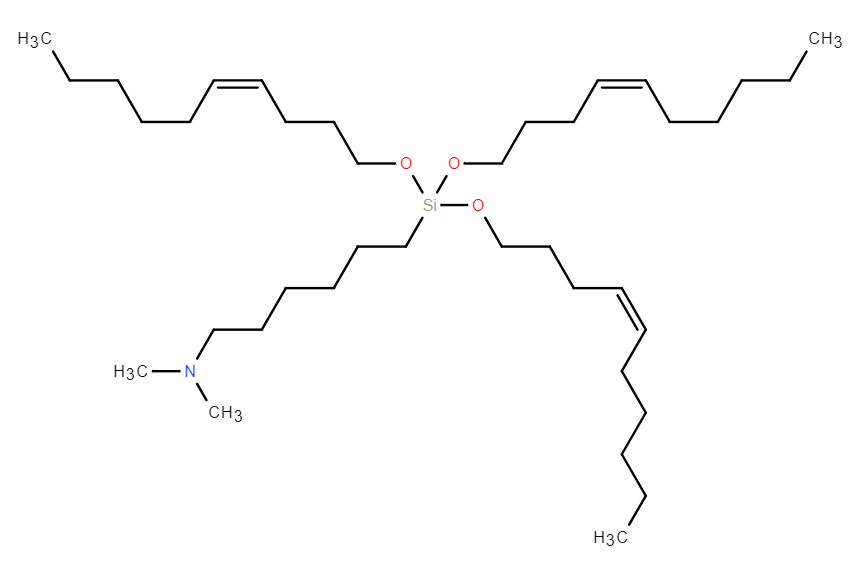
| DC67618 | GVS-18-B35 |
GVS-18-B35 is a leading silicon ether-based ionizable lipid that demonstrates exceptional performance in mRNA delivery. It features a biodegradable silyl ether linkage, which undergoes rapid, non-enzymatic hydrolysis, enabling near-complete clearance from the liver within 24 hours in both mice and non-human primates (NHPs). This degradation mechanism is independent of variable enzymatic activity, ensuring consistent pharmacokinetics across species. In vivo, GVS-18-B35 lipid nanoparticles (LNPs) achieve superior liver-specific protein expression with minimal off-target accumulation in the spleen, resulting in a high liver-to-spleen signal ratio and reduced immune stimulation. The LNPs exhibit excellent stability under frozen storage (-80°C) and maintain critical quality attributes, including particle size, polydispersity, and encapsulation efficiency, through multiple freeze-thaw cycles. With an optimal pKa (~6.3) and enhanced endosomal escape capability, GVS-18-B35 represents a robust and versatile platform for mRNA therapeutics, particularly suited for applications requiring frequent dosing due to its unique combination of high potency and rapid clearance profile.
More description
|
|
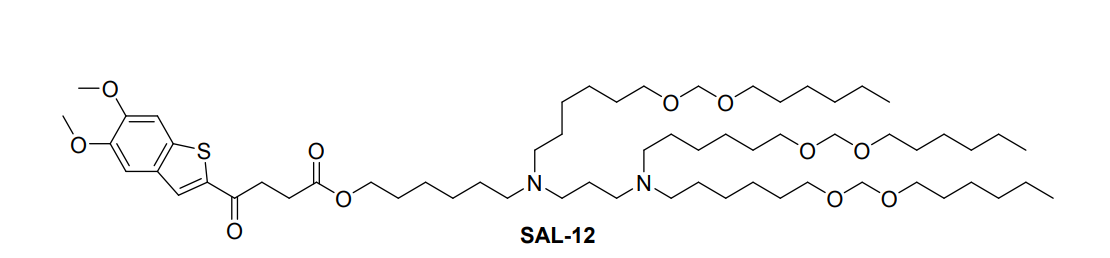
| DC67615 | STING Agonist Lipid SAL-12 |
SAL12 is a novel ionizable lipid derivative that integrates a non-nucleotide STING agonist (agonist 6) with an amino lipid tail through an ester bond, forming the core component of specialized lipid nanoparticles (SAL12-LNPs). These nanoparticles are designed for dual functionality: they efficiently encapsulate and deliver mRNA into dendritic cells while concurrently activating the STING pathway to stimulate innate immunity.
More description
|
|
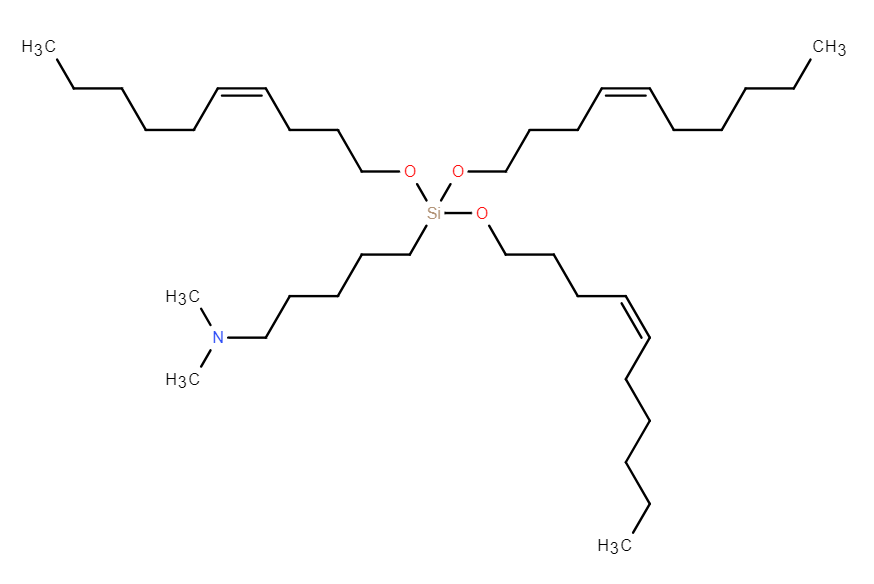
| DC67616 | GVS-18-B34 |
GVS-18-B34 is a highly potent, silicon ether-based ionizable lipid that enables efficient mRNA delivery via lipid nanoparticles (LNPs). Its key advantage lies in a biodegradable silyl ether linkage, which undergoes rapid, non-enzymatic hydrolysis, leading to near-complete clearance from the liver within 24 hours in both mice and non-human primates (NHPs). This degradation mechanism is species-agnostic, overcoming the variability associated with esterase-dependent lipids. In vivo, GVS-18-B34 LNPs demonstrated superior liver-specific protein expression and a high liver-to-spleen signal ratio, indicating minimal off-target accumulation and reduced immune stimulation compared to benchmarks like MC3 and SM-102. The LNPs exhibited excellent stability when stored frozen at -80°C, maintaining integrity over multiple freeze-thaw cycles. With its optimal pKa (~6.3) and efficient endosomal escape profile, GVS-18-B34 represents a promising candidate for therapeutic applications requiring frequent dosing, combining high potency with a favorable safety profile derived from its rapid clearance.
More description
|
|
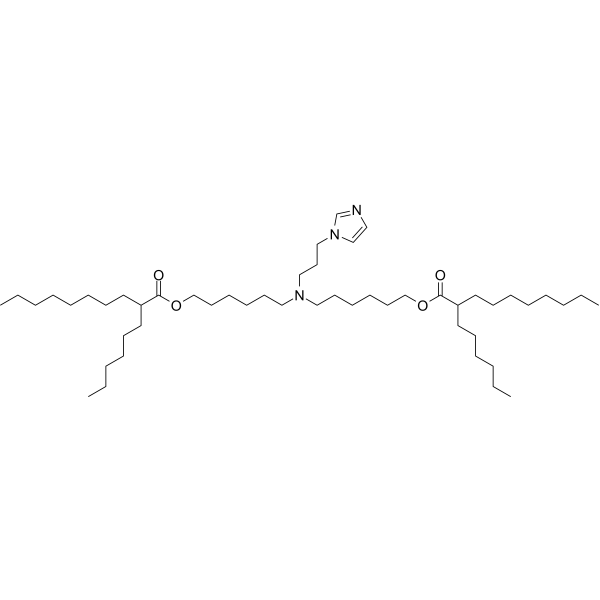
| DC82209 | ORNA Lipid 10a-26 Featured |
Lipid 10a-26 is an ionizable lipid developed by Orna Therapeutics for lipid nanoparticle (LNP) formulations. It features a biodegradable ester backbone and an ionizable headgroup, enabling efficient encapsulation and delivery of circular RNA (oRNA). Experimental data show that Lipid 10a-26 mediates robust protein expression in hepatocytes and immune cells (e.g., T cells), with strong liver-targeting specificity observed in vivo. Its optimized hydrolysis profile ensures stable oRNA delivery and reduced immunogenicity. For instance, LNPs formulated with Lipid 10a-26 (molar ratio 50:10:38.5:1.5) demonstrate high transfection efficiency in splenic B cells and sustained therapeutic protein production.The lipid’s design balances efficacy and safety, making it ideal for applications like CAR-T therapy and hepatic protein replacement.
More description
|
|
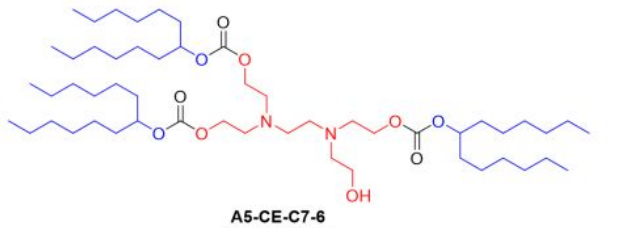
| DC67540 | Lipid A5-CE-C7-6 |
A5-CE-C7-6 is an ionizable lipid engineered for spleen-targeted mRNA delivery, integrating a hydroxylated dual-amine core (A5) for enhanced mRNA binding and endosomal escape, a biodegradable carbonate ester linker (CE) enabling rapid hydrolysis (61% degradation in 24 h), and branched heptyl hydrophobic tails (C7-6) that optimize nanoparticle stability and spleen tropism. When formulated into cholesterol-free lipid nanoparticles (B-8 formulation), its unique architecture—combining hydroxyl groups for cellular uptake, carbonate-mediated biodegradability, and branched-chain fluidity—achieves unprecedented efficiency: low pKa (~6.0) minimizes liver accumulation while enabling 21% transfection of splenic NK cells, outperforming benchmark systems like MC3 SORT LNPs by >10-fold in spleen-specific delivery and establishing a new standard for in vivo immune cell engineering.
More description
|
|
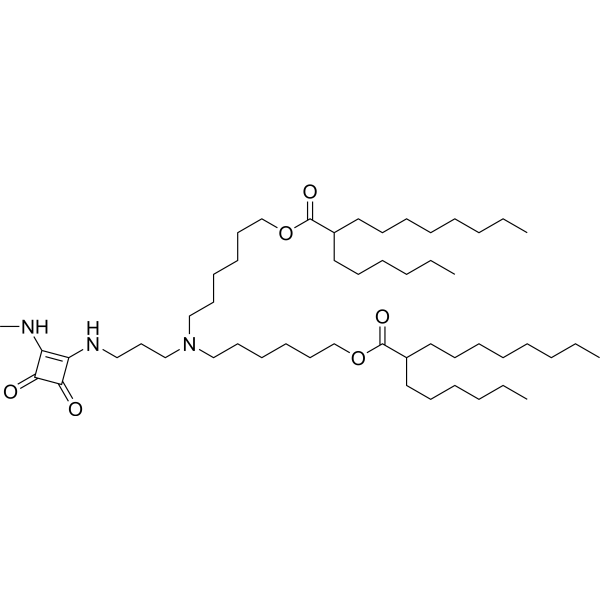
| DC67128 | Lipid 29 analogue-2(Lipid 16) Featured |
Lipid 29 analogue-2 is an ionizable lipid designed for the delivery of RNA-based therapeutics, such as mRNA or siRNA.
More description
|
|
| DC59126 | Genevant CL1 (lipid 10) Featured |
Genevant CL1 (lipid 10) is a novel ionizable lipid for rna delivery.Lipid 10 rapidly accumulated in the liver within the first hour of dosing (reflecting LNP uptake), but levels then steadily declined over the ensuing 2 weeks period, similar to MC3.Lipid 10 afforded more than double the expression of either approved lipid. We also observed high splenic expression for ALC-0315, which correlated with higher MCP-1 levels.Animals received a single 5 µg IM dose of LNP encapsulating firefly luciferase (fLuc) mRNA. Whole body imaging was performed 6 h later and expression at the injection site quantified. Lipid 10, ALC-0315, and SM-102 showed similar expression at the injection site, all greater than the older generation benchmarks lipids (DLinDMA, KC2, MC3). Lipid 10 and ALC-0315 also showed high expression in the liver, while SM-102 was less, and more similar to MC3.Lipid 10-based LNP reported similar anti-HA IgG titers to MC3 and ALC-0315 (Comirnaty) LNP, and higher than the SM-102 (SpikeVax) LNP composition. MCP-1 levels were generally similar, although the ALC-0315 composition had a significantly higher response at the 5 µg dose. All formulations reported good stability when stored frozen at −80 °C or at 2–8 °C for 1 month.
More description
|

|
| DC67291 | ATX054 Featured |
ATX-054 which is from Arcturus RNA delivery platform, is a novel ionizable lipid used in the formulation of lipid nanoparticles (LNPs) for the delivery of RNA.
More description
|
|
| DC58046 | C12-200 Featured |
C12-200 is a well-known cationic lipid used in the formulation of lipid nanoparticles (LNPs) for the delivery of therapeutic nucleic acids, including siRNA, mRNA, and CRISPR components. It is widely recognized for its high in vivo potency at low doses and is often used as a positive control ionizable lipid in research exploring new ionizable lipids.
More description
|

|
| DC31024 | SM-86 Featured |
SM86 is a cationic, ionizable lipid developed by Moderna as a core component of its lipid nanoparticle (LNP) platform for mRNA therapeutic delivery.SM-086 is structurally optimized and analogous to SM-102 (used in Moderna’s COVID-19 vaccines), with modifications aimed at enhancing mRNA delivery efficiency and safety.SM-86 serves as the primary cationic lipid in three investigational mRNA therapies targeting rare metabolic disorders:mRNA-3927: Restores propionyl-CoA carboxylase activity in propionic acidemia (PA).
mRNA-3705: Delivers methylmalonyl-CoA mutase mRNA for methylmalonic acidemia (MMA).
mRNA-3210: Provides phenylalanine hydroxylase mRNA to treat phenylketonuria (PKU).
More description
|

|
| DC31000 | LP-01 Featured |
LP-01 is an ionizable cationic amino lipid (pKa = ~6.1). It has been used in the generation of lipid nanoparticles (LNPs). LNPs containing LP-01 and encapsulating both Cas9 mRNA and modified single-guide RNA (sgRNA) for the transport protein transthyretin (Ttr) induce gene editing in liver cells in mice in a dose-dependent manner resulting in reduced serum Ttr levels for at least 12 months.
More description
|

|
| DC59217 | Arcturus lipid 2(ATX-0114) Featured |
Lipid 2,2(8,8) 4C CH3 is an ionizable cationic lipid (pKa = 6.69).1 It has been used in the generation of lipid nanoparticles (LNPs) for the delivery of siRNA in vivo. LNPs containing lipid 2,2(8,8) 4C CH3 and encapsulating siRNA targeting Factor VII decrease plasma Factor VII protein levels by 90% in mice.
More description
|

|
| DC57046 | ATX-126(ATX-0126, lipid 10p) Featured |
ATX-126(ATX-0126, 10p) is an ionizable cationic lipid (pKa = 6.38).It has been used in the generation of lipid nanoparticles (LNPs) for the delivery of siRNA. Intravenous administration of LNPs containing ATX-126(ATX-0126, 10p) and encapsulating Factor VII siRNA decrease Factor VII blood levels in mice.
More description
|

|
| DC67546 | ALC-0307 Featured |
ALC 0307 is an ionizable amino lipid developed by Acuitas Therapeutics, serving as the critical functional component in lipid nanoparticles (LNPs) for targeted therapeutic delivery. As the core cationic lipid in specific LNP formulations (e.g., k-abe for CPS1-Q335X correction), its key feature is pH-dependent chargeability: it remains neutral at physiological pH but becomes positively charged in acidic environments like endosomes. This property enables efficient encapsulation of nucleic acid payloads (>97% efficiency, e.g., base editor mRNA/gRNA complexes) and facilitates endosomal escape via membrane disruption post-cellular uptake. Its optimized structure promotes selective hepatocyte targeting by binding endogenous apolipoprotein E (ApoE), which subsequently interacts with LDL receptors on liver cells. Preclinical studies show rapid clearance (>99.5% plasma reduction in 14 days) and manageable transient toxicity (mild, reversible cytoplasmic vacuolation in hepatocytes, short-term ALT/AST elevation). LNPs containing ALC0307, alongside helper lipids (cholesterol, DSPC, and PEG-lipid ALC-0159), form stable ~73 nm particles with low polydispersity. This combination enables repeatable, liver-directed delivery of gene editing therapeutics with minimized off-target effects, underpinning its use in individualized in vivo gene correction therapies.
More description
|

|
| DC60683 | Lipid-168 Featured |
LIPID168(pKa ~6.5) is an optimized ionizable lipid engineered for in vivo mRNA delivery to hematopoietic stem cells (HSCs) in bone marrow. Developed by Yoltech Therapeutics through high-throughput screening of lipid libraries, it features a diethylamino head group and a tailored hydrophobic tail structure that enables antibody-free targeting. When Lipid 168 was formulated into lipid nanoparticles (LNPs), it achieved 48.5% base editing efficiency in bone marrow cells —surpassing benchmarks like LIPID-028 (19.7%)—and reduced off-target liver editing from 71% to 19% by incorporating miR-122 target sequences. In humanized β-thalassemia models, LNP 168 delivered ABE8e mRNA/sgRNA to patient-derived HSCs, yielding 42.6% editing at the HBG promoter, reactivating fetal hemoglobin (γ-globin) and rescuing erythroid defects . Its bone marrow specificity is driven by a unique protein corona enriched in albumin, fibronectin, and fibrinogen . Safety studies confirmed transient immune responses and no cumulative toxicity . LIPID-168 represents a promising non-viral platform for curative gene therapies in blood disorders.
More description
|

|
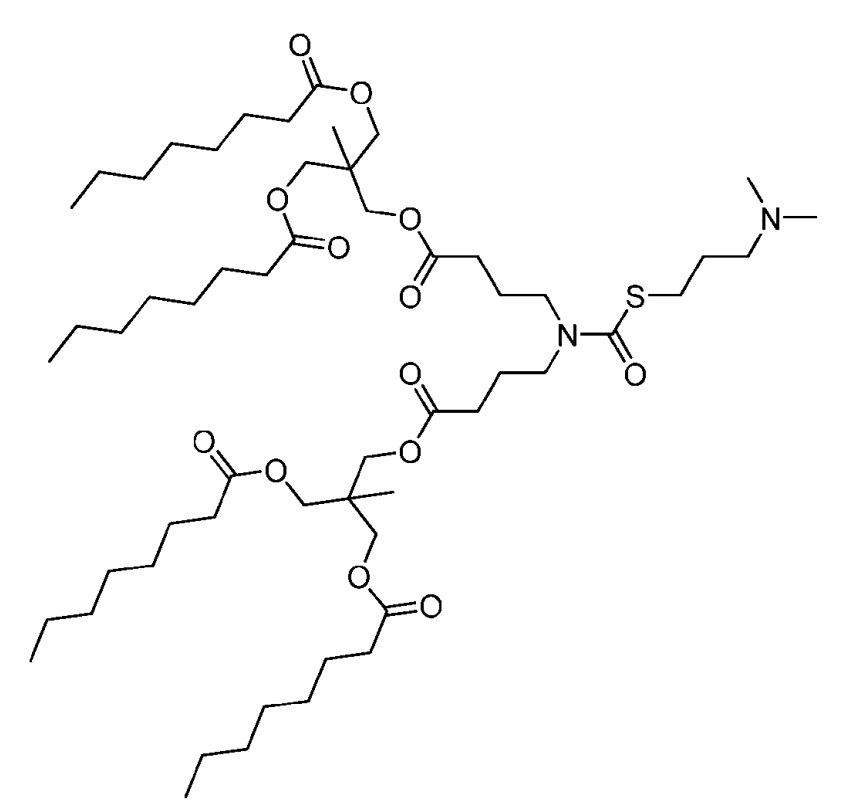
| DC67537 | DM3-BTA-14 Featured |
DM3-BTA-14 is a cationic lipid compound engineered for high-efficiency mRNA delivery developed by Hefei AlphaNA Biotechnology. Its structure features a rigid benzene-1,3,5-tricarboxamide core linked to a protonatable dimethylamino headgroup (-N(CH₃)₂) via a propylene spacer (-CH₂CH₂CH₂-) and two saturated C14 alkyl chains. This design enables ≈90% endosomal escape efficiency , superior lymph node targeting for vaccines , and effective tumor-specific mRNA delivery . It outperforms benchmark lipids while maintaining low cytotoxicity, forming stable nanoparticles with cholesterol/DSPC/DSPE-PEG (50:39:10:1 ratio) for therapeutic applications.
More description
|

|
| DC67536 | Lipid A1-EP10-O18A |
A1-EP10-O18A is an asymmetric ionizable lipid developed by Starna Therapeutics for mRNA vaccine delivery. Synthesized via Michael addition between amine alcohols and acrylates, its optimized structure—combining a hydrophilic C10 chain and hydrophobic unsaturated C18 tail—enables pH-dependent ionization. As the core component of the STAR0225 lipid nanoparticle (LNP) platform, it efficiently encapsulates mRNA and facilitates endosomal escape. Preclinical studies demonstrate superior in vivo mRNA delivery (vs. commercial SM102 LNPs), with enhanced local biodistribution and minimal off-target accumulation. This lipid underpins STR-V003, an RSV prefusion F mRNA vaccine showing robust immunogenicity and protection in animal models, supporting its clinical transition (NCT06344975).
More description
|

|
| DC60856 | DMA4-H228 Featured |
DMA4-H228 is a novel, biodegradable lipidoid specifically engineered for spleen-targeted mRNA delivery. Its structure combines a dimethylamino (DMA4) headgroup with a unique hyperbranched lipid tail (H228) synthesized via Michael addition, incorporating ester bonds for enhanced biodegradability. This design enables the formation of stable lipid nanoparticles (LNPs) (~170 nm) with high mRNA encapsulation efficiency (>96%).
Critically, DMA4-H228 exhibits exceptional intrinsic tropism for the spleen (>98% targeting efficiency after IV administration), requiring no external targeting ligands. It selectively delivers mRNA to splenic antigen-presenting cells (APCs), including dendritic cells, macrophages, and B cells. This triggers potent immune activation: rapid IFNα secretion, upregulation of APC maturation markers (CD86/CD40), and robust antigen-specific immune responses.
Demonstrating significant therapeutic potential, DMA4-H228-based mRNA vaccines effectively inhibit tumor growth in melanoma models (e.g., B16F10-OVA). This correlates with increased tumor-infiltrating CD8⁺ T cells, a shift towards pro-inflammatory M1 macrophages, elevated antigen-specific antibodies (IgG), and strong T cell responses (evidenced by IFNγ⁺ spots). Its ability to bypass liver tropism and directly activate splenic APCs makes DMA4-H228 a powerful platform for next-generation mRNA vaccines and cancer immunotherapy.
More description
|

|
| DC67515 | CICL-207 Featured |
CICL 207 is structurally optimized based on Lipid CICL-1. CICL207 is a constrained ionizable cationic lipid designed for lipid nanoparticle (LNP) delivery systems developed by Capstan. Its structure features a rigid cyclic backbone (e.g., pyrrolidine-derived core) paired with a tertiary amine group that ionizes at acidic pH (pKa ~6.5–7.0), enhancing endosomal escape. The lipid includes asymmetric hydrophobic tails (likely C14–C18 alkyl/ester chains) to stabilize LNP membranes and improve nucleic acid encapsulation. Integrated into LNPs (e.g., 58% CICL-207, 10% DSPC, 30.5% cholesterol, PEG-lipids), it enables targeted delivery to T cells (anti-CD5/CD8 tLNPs) with high transfection efficiency (spleen T cells >70% mCherry+), reduced liver uptake, and low toxicity (no significant ALT/AST elevation in rats). Its constrained design balances stability, tissue specificity, and biocompatibility for gene therapy applications.CICL 207 (F50) significantly outperforms CICL-1 by delivering dramatically enhanced target cell transfection with reduced off-target effects. It achieves >50% transfection efficiency in splenic T-cells—nearly double that of CICL-1—while slashing off-target expression in liver cells to <5% (versus >15% for CICL-1. This precision translates to superior therapeutic outcomes: CICL-207 enables ~95% B-cell depletion in CAR-T applications, far exceeding CICL-1 ’s ~60% efficacy. Critically, it maintains an exceptional safety profile, showing no significant liver toxicity or inflammatory cytokine elevation even at high doses. Furthermore, CICL-207 demonstrates 2-fold higher transfection efficiency in hematopoietic stem cells, enabling robust gene editing. Its optimized pKa (~6.5) and constrained amine structure enhance endosomal escape while minimizing Kupffer cell uptake, making it ideal for targeted therapeutics requiring both potency and safety.
More description
|

|
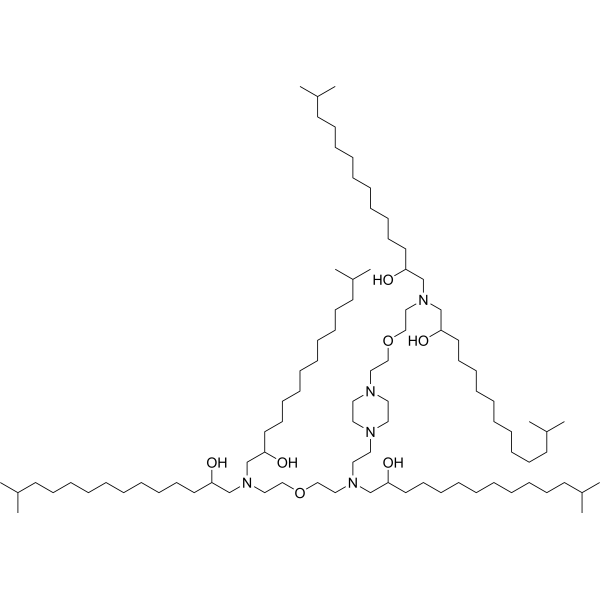
| DC13101 | E10i-494 Featured |
E10i-494 is a branched ionizable lipid designed to enhance the delivery of mRNA and CRISPR-Cas9 ribonucleoprotein (RNP) complexes. It belongs to the Branched Endosomal Disruptor (BEND) lipid family, which features terminal branching to improve endosomal escape and cellular uptake.E10i-494 demonstrated exceptional performance in T cell engineering, achieving >80% transfection efficiency in primary human T cells. This is significantly higher than the ~70% efficiency achieved by the linear lipid C14-494.The isopropyl branch enhances the lipid's ability to penetrate and disrupt endosomal membranes, leading to improved release of mRNA and RNPs into the cytoplasm.Despite its high efficiency, E10i-494 exhibits low cytotoxicity, making it suitable for therapeutic applications.E10i-494 is particularly effective for delivering mRNA to T cells, making it a promising tool for CAR-T cell therapy and other immunotherapies.Its ability to deliver CRISPR-Cas9 RNPs efficiently also makes it suitable for in vivo gene editing applications.
More description
|
|
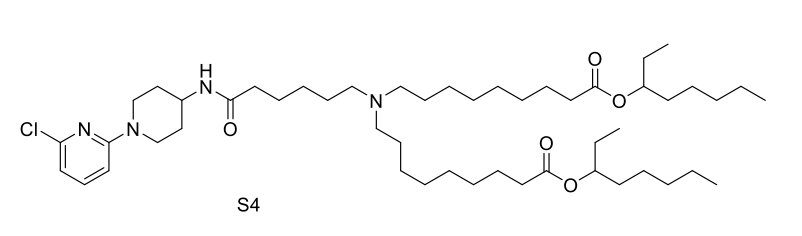
| DC67569 | Lipid S4 Featured |
Lipid S4 is an advanced ionizable lipid engineered for systemic mRNA delivery to the brain, leveraging SR-57227—a high-affinity 5-HT3 receptor ligand—as its core head group to enable targeted blood-brain barrier (BBB) penetration via receptor-mediated transcytosis, while incorporating amino linkers for pH-responsive ionization and biodegradable branched ester tails to facilitate efficient endosomal escape and intracellular mRNA release; optimized through orthogonal screening into OS4 LNP (formulated at S4/DOPE/Chol/DMG-PEG2k = 40:40:60:0.75 molar ratio), it demonstrated a 13.3-fold increase in brain mRNA expression compared to FDA-approved MC3 LNPs, and further conjugation with the Tat cell-penetrating peptide yielded OS4T LNP, boosting delivery efficiency by 12.7-fold over OS4 alone and enabling broad mRNA expression across neurons, astrocytes, microglia, and endothelial cells; validated in orthotopic glioblastoma models, OS4T delivered engineered IL-12 mRNA, suppressing tumor growth and extending median survival to 37 days (vs. 17 days for controls) with minimal systemic toxicity, positioning S4-based LNPs as a robust, translatable platform for CNS-targeted therapeutics.
More description
|
|
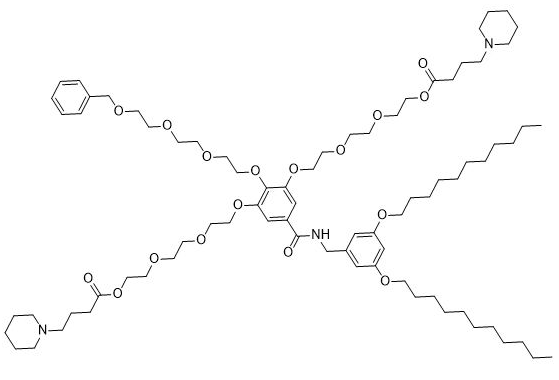
| DC67292 | IAJD34 Featured |
IAJD-34 is a one-component ionizable amphiphilic Janus dendrimer specifically engineered for targeted mRNA delivery to the lung parenchyma, as described by Meshanni et al. in Nature Communications article "Targeted delivery of TGF-β mRNA to murine lung parenchyma using one-component ionizable amphiphilic Janus Dendrimers" . This synthetic nanoparticle self-assembles with mRNA through simple mixing in acetate buffer, forming stable dendrimersomes approximately 93-97 nm in size with high encapsulation efficiency (>95%) and a positive zeta potential (~48 mV). Its defining feature, highlighted in the study, is exceptional lung tropism after intravenous injection, enabling significantly higher luciferase expression in murine lungs compared to other organs. As demonstrated by Meshanni et al., IAJD 34 effectively delivers therapeutic mRNA (e.g., TGF-β mRNA) to the lower lung, inducing transient protein production with minimal systemic toxicity at appropriate doses (e.g., 10 µg), offering a promising strategy for treating parenchymal lung diseases.
More description
|
|
| DC60211 | TCL053 Featured |
TCL053 is an ionizable amino lipid.1 It has been used in the generation of lipid nanoparticles (LNPs) and has a pKa value of 6.8. LNPs containing TCL053 and encapsulating mRNA encoding the Cas9 nuclease, in combination with LNPs containing TCL053 and encapsulating single-guide RNA (sgRNA) targeting the Rosa26 locus, have been used to induce CRISPR-mediated gene editing in the mouse gastrocnemius muscle.TCL053 is an ionizable lipid that has received FDA approval
for preparing mRNA vaccines. It is a three-tailed ionizable lipid
to overcome the disadvantage of nonrepeatable administration of
AAV vectors. In addition, combined with limb perfusion administration,
TCL053 iLNPs could transiently deliver CRISPR-Cas9
mRNA and sgRNA to multiple muscle tissues, reducing
immunogenicity and increasing the safety of iLNPs. It is
great progress for treating Duchenne muscular dystrophy and
other diseases that require multiple doses.
More description
|

|
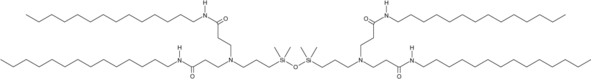
| DC60663 | Si5-N14 Featured |
Si5-N14 is a lipid-based molecule engineered with siloxane groups, designed specifically for efficient mRNA delivery to the lungs. The incorporation of siloxane units boosts the cellular uptake of mRNA-loaded lipid nanoparticles (LNPs) and enhances their ability to escape from endosomes. These properties significantly increase the overall effectiveness of mRNA delivery, making Si5-N14 a promising tool for targeted therapeutic applications.
More description
|
|
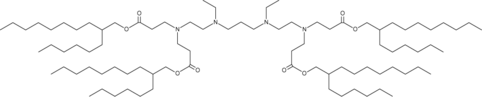
| DC60546 | 514O6,10 Featured |
514O6,10 is an ionizable lipidoid. 514O6,10 formulated LNPs facilitate mRNA delivery to the pancreas.
More description
|
|
| DC70010 | 98N12-5 Featured |
98N12-5 is an ionizable cationic lipid. It has been used in combination with other lipids in the generation of lipid nanoparticles (LNPs). LNPs containing 98N12-5 and encapsulating proprotein convertase subtilisin kexin type 9 (PCSK9) siRNA selectively accumulate in the liver and reduce total serum cholesterol levels in mice and rats and serum LDL levels in cynomolgus monkeys.
More description
|

|