 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
| Cat. No. | Product Name | Field of Application | Chemical Structure |
|---|---|---|---|
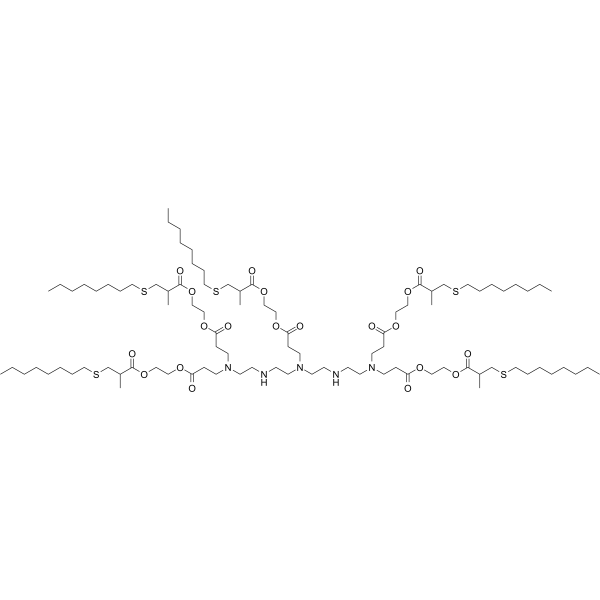
| DC49907 | 5A2-SC8 Featured |
5A2-SC8 is a dendrimer for miRNA delivery to late-stage liver tumors with low hepatotoxicity. 5A2-SC8 shows potent EC50 < 0.02 mg/kg (siRNA against FVII (siFVII)) in dose-response experiments, and well tolerated in separate toxicity studies in chronically ill mice bearing MYC-driven tumors. 5A2-SC8 is a degradable lipid-like compound (ester-based dendrimer) for small RNAs delivery.5A2-SC8, was obtained by screening a large library of more than 1500 ester-based dendrimers
containing ionizable amino groups, which have three
tertiary amine heads and five lipid tails. Based on this library,
the in vitro transfection efficiency of different formulations of
5A2-SC8 iLNPs was evaluated, discovering the optimal formulation
(5A2-SC8, DOPE, cholesterol, PEG at a molar ratio of
15:15:30:3) of 5A2-SC8 iLNPs for delivering fumarylacetoacetate
hydrolase (FAH) mRNA to liver.After the intravenous injection
via tail, the model mice of hepatorenal tyrosinemia type I
had strong FAH protein expression, which prevented
body weight loss and increased the survival rate of hepatorenal
tyrosinemia mice . In addition to introducing utility of
5A2-SC8 iLNPs for the therapeutic intervention, the 5A2-SC8
iLNPs containing DOTAP have been used to establish complex
mouse models via intravenous injection, including in situ liverspecific
cancer model and in situ lung-specific cancer model.
Based on this iLNPs delivery system, 5A2-SC8 induced model
construction method overcomes the time-consuming and costly
disadvantages of traditional animal models establishing methods,
including transgenesis and gene engineering in embryonic
stem cells.
More description
|
|
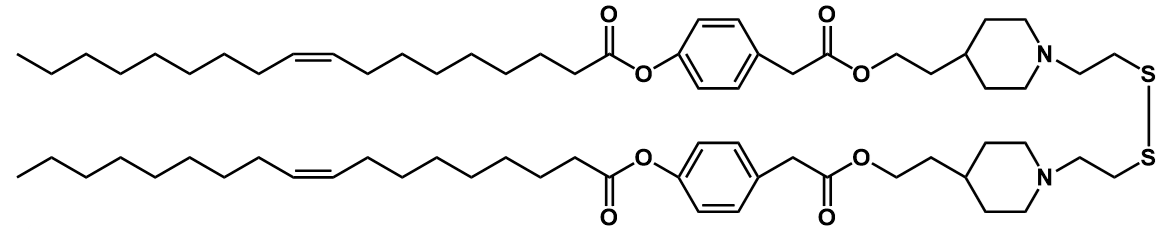
| DC59002 | ssPalmO-Phe Featured |
ssPalmO-Phe(SS-OP) is a self-degradable material for the delivery of oligonucleotides. ssPalmO-Phe is a self-degradable derivative of ssPalm that is self-degraded in the intraparticle space by a specific hydrolytic reaction. ssPalmO-Phe is beneficial for overcoming the plasma/endosomal membrane, LNP-ssPalmO-Phe can be used to deliver both nucleic acids.
More description
|
|
| DC31000 | LP-01 Featured |
LP-01 is an ionizable cationic amino lipid (pKa = ~6.1). It has been used in the generation of lipid nanoparticles (LNPs). LNPs containing LP-01 and encapsulating both Cas9 mRNA and modified single-guide RNA (sgRNA) for the transport protein transthyretin (Ttr) induce gene editing in liver cells in mice in a dose-dependent manner resulting in reduced serum Ttr levels for at least 12 months.
More description
|

|
| DC82301 | IC-8(lipid MIC5) Featured |
IC8 is an ionizable cationic lipid. It has been used in combination with other lipids for the formation of lipid nanoparticles (LNPs). Immunization with severe acute respiratory coronavirus 2 (SARS-CoV-2) spike glycoprotein mRNA in IC8- and manganese-containing LNPs induces IgG responses to SARS-CoV-2 Delta and Omicron variants in mice.1 Administration of mRNA encoding B7-H3 X CD3 bispecific T cell engaging (BiTE) antibodies in IC8-containing LNPs reduces tumor growth in MV4-11 and A375 mouse xenograft models.
More description
|

|
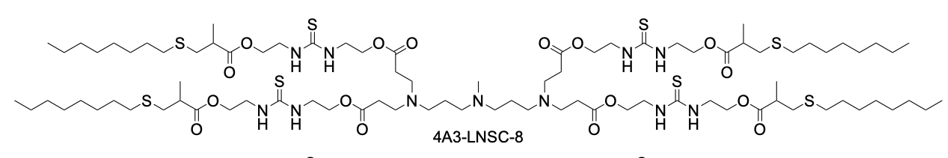
| DC67990 | 4A3-LNSC8 Featured |
4A3-LNSC8 is a strategically designed thiourea-functionalized ionizable lipid that serves as the foundational core for a novel anion-coordination delivery platform. Its structure features a central 4A3 amine headgroup symmetrically extended with four hydrophobic tails, each incorporating a biodegradable ester linkage and a key thiourea-bridged linker. The inclusion of the thiourea group is the pivotal innovation, as it provides specific hydrogen-bonding sites capable of interacting with various halide anions (F⁻, Cl⁻, I⁻). When formulated into lipid nanoparticles (LNPs) without anion coordination, 4A3-LNSC8 itself exhibits a characteristic liver tropism, efficiently delivering mRNA to hepatocytes following systemic administration, with a measured surface pKa of approximately 5.54. However, its primary significance lies in its role as a versatile precursor. The strong anion-binding capability of its thiourea linkers allows for predictable modulation of the LNP's properties. Upon binding with anions like Cl⁻, the resulting complex (e.g., Cl-4A3-LNSC8) undergoes a significant pKa shift, which reprograms the LNP's in vivo fate, redirecting mRNA delivery from the liver to secondary lymphoid organs such as the spleen and lymph nodes. Thus, 4A3-LNSC8 is not merely an efficient ionizable lipid but a programmable scaffold that enables precise control over organ-targeting specificity through simple anion coordination, offering a powerful rational design strategy for advanced mRNA therapeutics.
More description
|
|
| DC53130 | 93-O17S Featured |
93-O17S is an imidazole-based synthetic lipidoid for in vivo mRNA delivery. Lipid nanoparticles (LNPs) with 93-O17S promotes both the cross-presentation of tumor antigens and the intracellular delivery of cGAMP (STING agonist).
More description
|

|
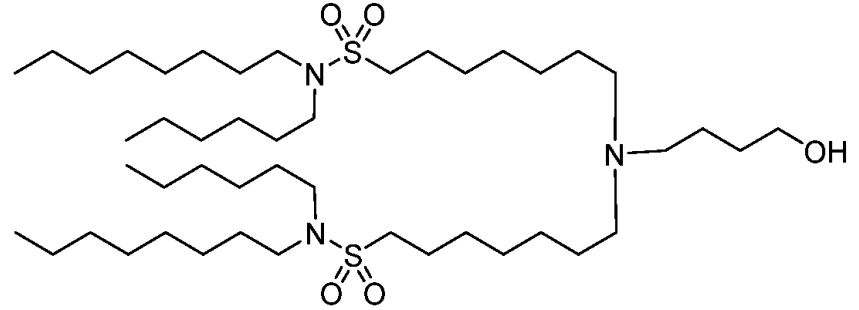
| DC67281 | BNT-51 Featured |
BNT-51 is an ionizable thiolipid developed by Biontech, characterized by its sulfur-containing moieties and a multiarm dendron-like architecture. Synthesized via reactions between amine-containing compounds and sulfur-based halides or sulfonates, it forms stable lipid nanoparticles (LNPs) optimized for mRNA delivery. The LNPs exhibit uniform particle size (80–100 nm, PDI <0.2), near-neutral zeta potential, and high mRNA encapsulation efficiency (>90%), while maintaining payload integrity through freeze-thaw cycles and extended storage. In vitro, BNT-51 demonstrates low cytotoxicity (>80% cell viability in C2C12, HepG2, and HEK293 cells) and superior transfection efficiency compared to conventional lipids, particularly in immune cells such as CD4+/CD8+ T cells within PBMCs. Its modular design allows integration of stealth lipids (e.g., PEG or vitamin E derivatives) to prolong circulation time and minimize immune activation, as evidenced by low hemolysis and complement activation risks. In vivo, BNT-51-based LNPs enable targeted mRNA delivery to splenic macrophages, achieving potent genome editing (e.g., Cre mRNA) and therapeutic protein expression (e.g., BACH1) in preclinical models. With its tunable structure, robust stability, and cell-specific tropism, BNT-51 holds promise for advancing mRNA therapeutics in gene editing, cancer immunotherapy, and regenerative medicine, offering a versatile platform for next-generation nanomedicine.
More description
|
|
| DC67991 | Lenalidomide-acetamido-O-PEG3-C2-azide Featured |

|
|
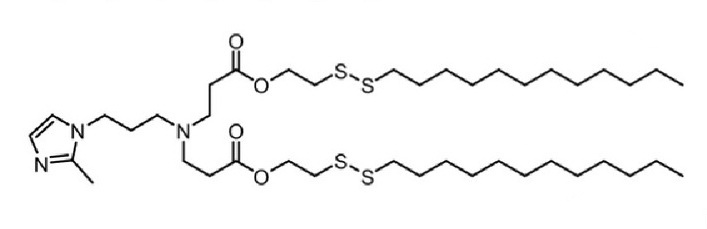
| DC60495 | 9322-O16B Featured |
9322-O16B is a lipidoid for the efficient delivery of antiCD19 mRNA CAR to murine primary macrophages. LNP 9322-O16B is more efficient than delivery with lipofectamine 2000 (LPF2K) or MC3.
More description
|
|
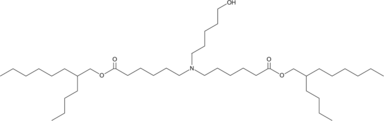
| DC67450 | A28-C6B2 |
A28-C6B2 is an ionizable lipid (pKa 6.43) designed for mRNA encapsulation in lipid nanoparticles (LNPs). Following intravenous injection in mice, these LNPs exhibit spleen-selective accumulation, particularly localizing in F4/80+ macrophages and CD11c+ dendritic cells, with moderate uptake by T lymphocytes.
More description
|
|
| DC80065 | 113-O12B Featured |
113-O12B is a disulfide bond-containing ionizable cationic lipidoid. 113-O12B LNP, an LN-targeting LNP delivery system, is developed for a mRNA cancer vaccine. The 113-O12B/mRNA shows enhanced expression in APCs compared with ALC-0315/mRNA, indicating the LN-specific targeting ability.
More description
|
.gif)
|
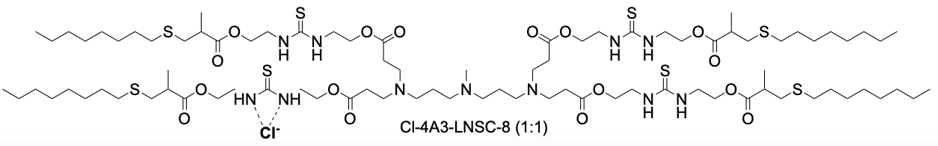
| DC67989 | Cl-4A3-LNSC8 Featured |
Cl-4A3-LNSC8 represents a novel class of thiourea-functionalized ionizable lipids engineered for selective organ-targeted mRNA delivery. Its core innovation lies in an anion-coordination strategy, where the parent lipid, 4A3-LNSC8, binds chloride ions (Cl⁻) via hydrogen-bonding interactions with its thiourea groups. This binding event is not merely structural but functionally critical, as it induces a significant shift in the surface pKa of the resulting lipid nanoparticles (LNPs) from approximately 5.54 to 8.79. This pKa modulation is the key mechanism that redirects the organotropism of the LNPs upon systemic administration. While the unmodified 4A3-LNSC8 LNPs preferentially deliver mRNA to the liver, Cl-4A3-LNSC8 LNPs effectivelyreprogram this tropism, enabling highly efficient mRNA delivery to secondary lymphoid organs (SLOs), particularly the spleen and lymph nodes. This platform demonstrates remarkable efficacy, achieving up to 65.7% gene editing efficiency in splenic macrophages in vivo, significantly outperforming benchmark delivery systems. Furthermore, by leveraging the coordination with different halides, such as iodine for computed tomography (CT) contrast, the system can be adapted for dual-modal theranostic applications, enabling simultaneous lymphatic metastasis imaging and therapeutic mRNA delivery.
More description
|
|
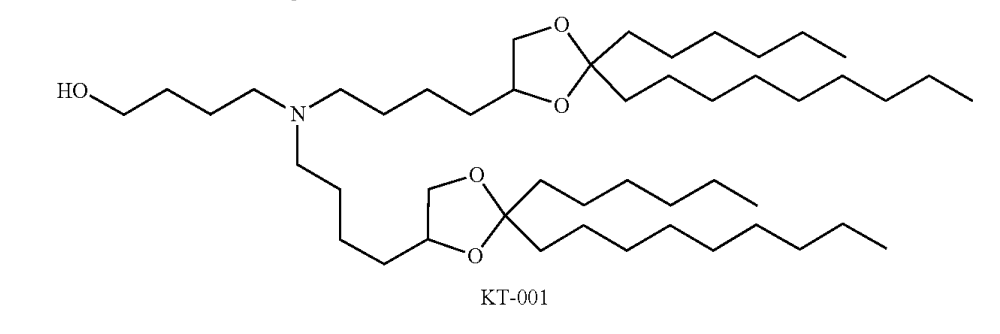
| DC67988 | KT-001 Featured |
KT-001 is a novel ionizable cationic lipid disclosed in patent US 2026/0007612 A1
More description
|
|
| DC70647 | Neurounina-1 Featured |
Neurounina-1 is a small molecule Na+/Ca2+ exchanger (NCX) activator, stimulates NCX1 and NCX2 activities with EC50 of 1.1-2.7 nM, does not affect NCX3 activity.Neurounina-1 (10 nM) reduced cell death of primary cortical neurons exposed to oxygen-glucose deprivation followed by reoxygenation, also reduced γ-aminobutyric acid (GABA) release, enhanced GABA(A) currents, and inhibited both glutamate release and N-methyl-d-aspartate receptors in vitro.Neurounina-1 effectively protects against stroke damage in vivo.
More description
|
|
| DC74057 | KZR-8445 Featured |
KZR-8445 (KZR8445) is a cyclic depsipeptide, client-selective Sec61 inhibitor with IC50 of 100 nM, targets the Sec61 translocon and selectively modulate Sec61-mediated protein biogenesis.
More description
|
.png)
|
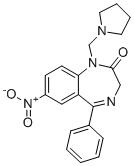
| DC10678 | fluticasone Featured |
Fluticasone is an inhaled corticosteroid used for respiratory research. Fluticasone is a Smo agonist with an IC50 value of 99 nM. Fluticasone activates Hedgehog signaling and promotes the proliferation of primary neuronal stem or precursor cells.
More description
|
|
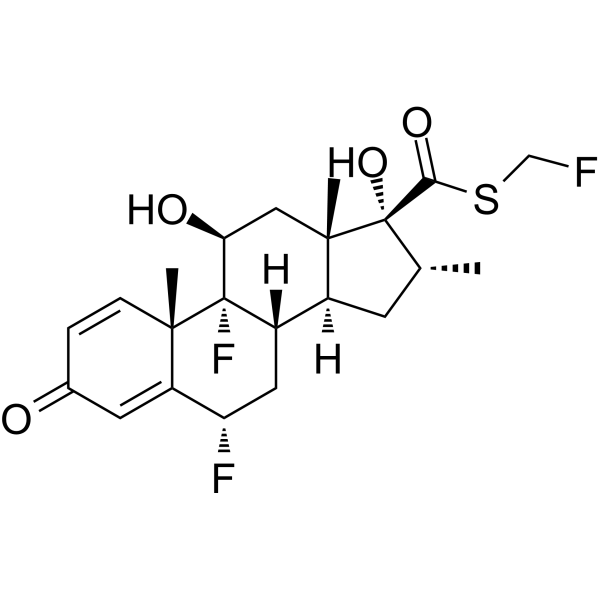
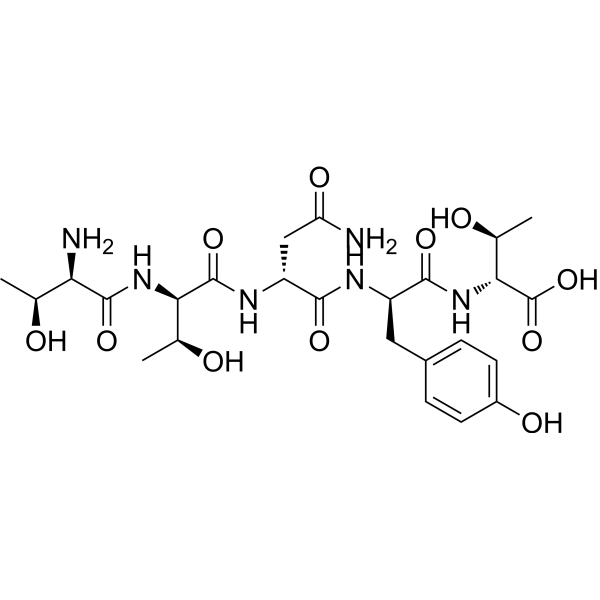
| DC73404 | RAP-103 Featured |
RAP-103 is an orally active, stabilized pentapeptide analog of DAPTA (D-ala-peptide T-amide), and multi-chemokine receptor antagonist.
More description
|
|
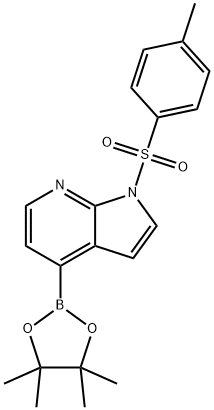
| DC67767 | 1-TOSYL-1H-PYRROLO[2,3-B]PYRIDINE-4-BORONIC ACID PINACOL ESTER Featured |
|
|
| DC67766 | 3-(2-aminoethoxy)propanoic acid hydrochloride Featured |
propanoic acid hydrochloride.gif)
|
|
| DC67765 | 3-(Aminomethyl)-6-methyl-4-propylpyridin-2(1H)-one Featured |
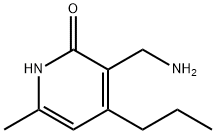
|
|
| DC67764 | tert-butyl (2S)-2-(cyanomethyl)-4-[2-[[(2S)-1-methylpyrrolidin-2-yl]methoxy]-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl]piperazine-1-carboxylate Featured |
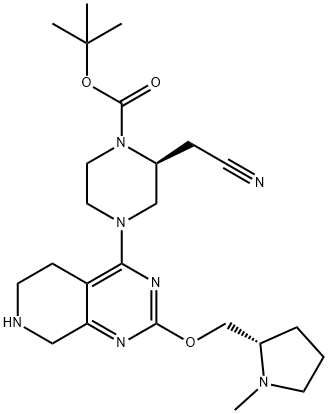
|
|
| DC67763 | methyl (2S,4R)-1-[(2S)-2-[(1-fluorocyclopropyl)formamido]-3,3-dimethylbutanoyl]-4-hydroxypyrrolidine-2-carboxylate Featured |
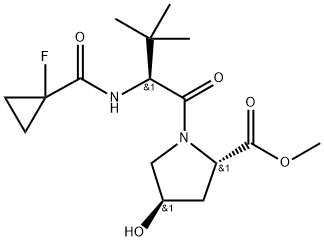
|
|
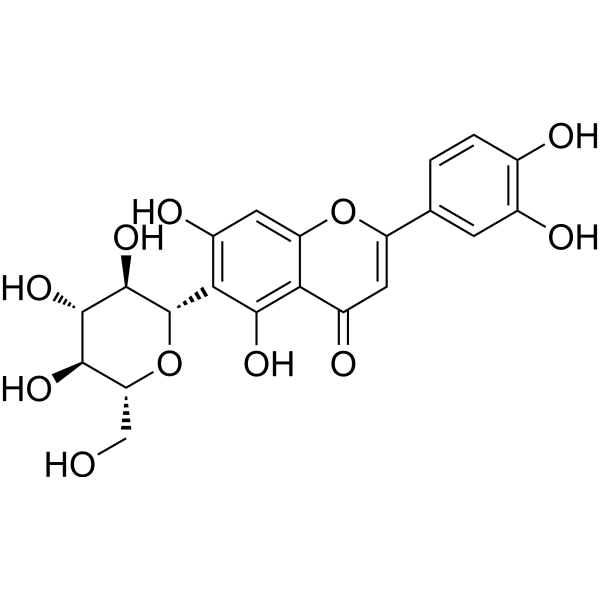
| DCY-132 | Homoorientin Featured |
Homoorientin is a potent inhibitor of COX-2 with an IC50 value of 39 μM.
More description
|
|
| DC67761 | tert-butyl (3S)-3-(cyanomethyl)piperazine-1-carboxylate Featured |
-3-(cyanomethyl)piperazine-1-carboxylate.gif)
|
|
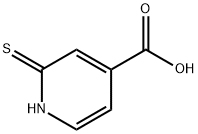
| DC67759 | 2-Mercaptopyridine-4-carboxylic acid Featured |
|
|
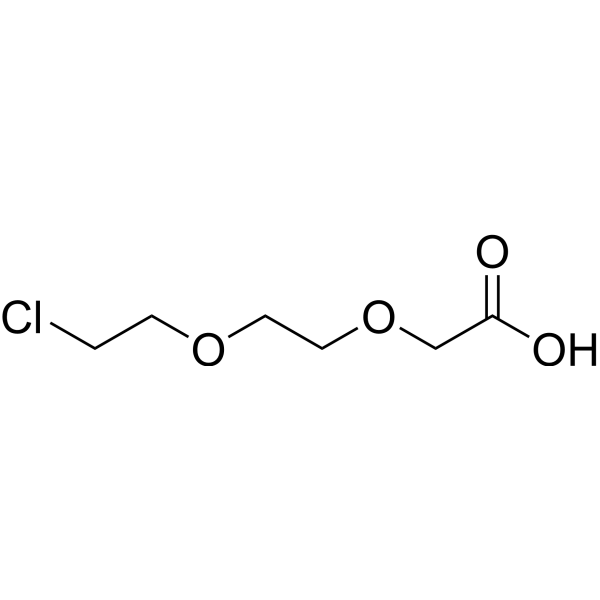
| DC67987 | Cl-PEG2-acid Featured |
Cl-PEG2-acid is a PEG-based PROTAC linker that can be used in the synthesis of PROTACs.
More description
|
|
| DC67758 | 3-(Fluoromethyl)azetidine hydrochloride Featured |
azetidine hydrochloride.gif)
|
|
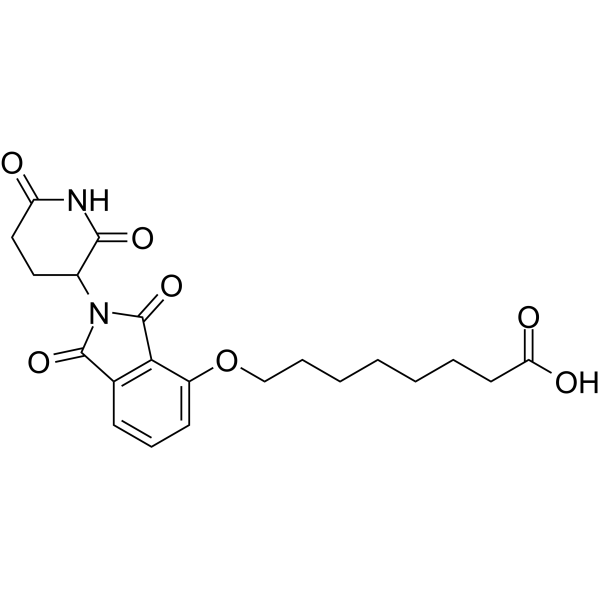
| DC67986 | Thalidomide-O-C7-acid Featured |
Thalidomide-O-C7-acid is a synthesized E3 ligase ligand-linker conjugate that incorporates the Thalidomide (HY-14658) based cereblon ligand and a linker used in PROTAC technology.
More description
|
|
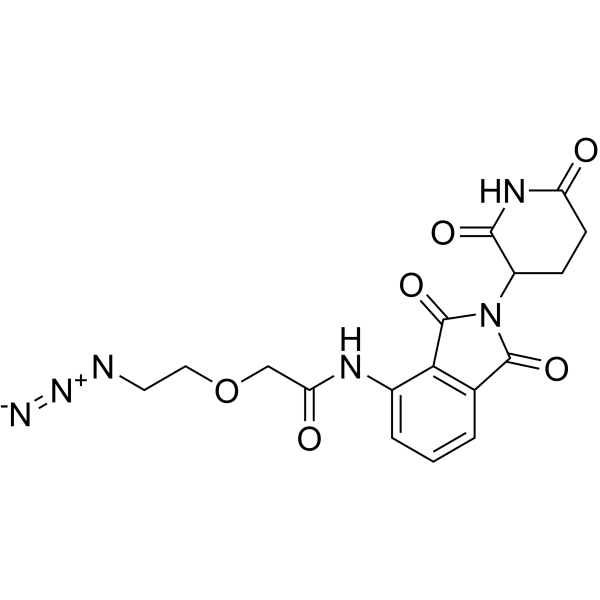
| DC67757 | Pomalidomide-PEG1-azide Featured |
Pomalidomide-PEG1-azide is a E3 ligase lgand-linker conjugate. Pomalidomide-PEG1-azide incorporates the Pomalidomide based cereblon ligand and a linker. Pomalidomide-PEG1-azide can be used to design a PROTAC BRD4 Degrader-1 (HY-133131). Pomalidomide-PEG1-azide is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
More description
|
|
| DC67756 | (S,R,S)-AHPC-PEG3-N3 Featured |
(S,R,S)-AHPC-PEG3-N3 is a synthesized E3 ligase ligand-linker conjugate that incorporates the (S,R,S)-AHPC based VHL ligand and 3-unit PEG linker used in PROTAC technology. (S,R,S)-AHPC-PEG3-N3 is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
More description
|
-AHPC-PEG3-N3.gif)
|